Page 1217 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1217
Chapter 67 Chronic Myeloid Leukemia 1063
A A
100 100
90 90
80 Complete hematologic response 80
Rates of response (%) 60 Complete cytogenetic response Patients without progression (%) 60 Response at 12 months
70
70
Major cytogenetic response
50
50
40
40
30
20
Partial cytogenetic response
20 30 Complete cytogenetic response
10 No major cytogenetic response
10
0
0 0 12 24 36 48 60 72
0 6 12 18 24 30 36 42 48 54 60 66
Months Months
B
100 B
100
90 Progression All events 90
80
Patients without event (%) 70 Patients without progression (%) 70 Complete cytogenetic response
80
60
60
50
50
Response at 18 months
40
40
30
20
Complete cytogenetic response
20
with <3 log reduction
10 30 with ≥3 log reduction
10 No complete cytogenetic response
0
0 12 24 36 48 60 72 0
0 12 24 36 48 60 72
Months
No. of events Months
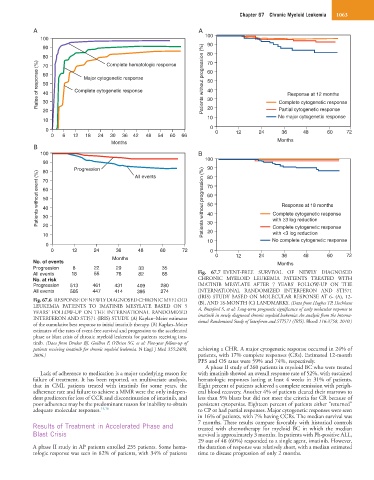
Progression 8 22 29 33 35
All events 18 55 76 82 85 Fig. 67.7 EVENT-FREE SURVIVAL OF NEWLY DIAGNOSED
No. at risk CHRONIC MYELOID LEUKEMIA PATIENTS TREATED WITH
Progression 513 461 431 409 280 IMATINIB MESYLATE AFTER 7 YEARS’ FOLLOW-UP ON THE
All events 505 447 414 395 274 INTERNATIONAL RANDOMIZED INTERFERON AND STI571
(IRIS) STUDY BASED ON MOLECULAR RESPONSE AT 6- (A), 12-
Fig. 67.6 RESPONSE OF NEWLY DIAGNOSED CHRONIC MYELOID (B), AND 18-MONTH (C) LANDMARKS. (Data from Hughes TP, Hochhaus
LEUKEMIA PATIENTS TO IMATINIB MESYLATE BASED ON 5 A, Branford S, et al: Long-term prognostic significance of early molecular response to
YEARS’ FOLLOW-UP ON THE INTERNATIONAL RANDOMIZED imatinib in newly diagnosed chronic myeloid leukemia: An analysis from the Interna-
INTERFERON AND STI571 (IRIS) STUDY. (A) Kaplan–Meier estimates tional Randomized Study of Interferon and STI571 (IRIS). Blood 116:3758, 2010.)
of the cumulative best response to initial imatinib therapy. (B) Kaplan–Meier
estimates of the rates of event-free survival and progression to the accelerated
phase or blast crisis of chronic myeloid leukemia for patients receiving ima-
tinib. (Data from Druker BJ, Guilhot F, O’Brien SG, et al: Five-year follow-up of
patients receiving imatinib for chronic myeloid leukemia. N Engl J Med 355:2408, achieving a CHR. A major cytogenetic response occurred in 24% of
2006.) patients, with 17% complete responses (CRs). Estimated 12-month
PFS and OS rates were 59% and 74%, respectively.
A phase II study of 260 patients in myeloid BC who were treated
Lack of adherence to medication is a major underlying reason for with imatinib showed an overall response rate of 52%, with sustained
failure of treatment. It has been reported, on multivariate analysis, hematologic responses lasting at least 4 weeks in 31% of patients.
that in CML patients treated with imatinib for some years, the Eight percent of patients achieved a complete remission with periph-
adherence rate and failure to achieve a MMR were the only indepen- eral blood recovery. Another 4% of patients cleared their marrows to
dent predictors for loss of CCR and discontinuation of imatinib, and less than 5% blasts but did not meet the criteria for CR because of
poor adherence may be the predominant reason for inability to obtain persistent cytopenias. Eighteen percent of patients either “returned”
adequate molecular responses. 15,16 to CP or had partial responses. Major cytogenetic responses were seen
in 16% of patients, with 7% having CCRs. The median survival was
Results of Treatment in Accelerated Phase and 7 months. These results compare favorably with historical controls
treated with chemotherapy for myeloid BC in which the median
Blast Crisis survival is approximately 3 months. In patients with Ph-positive ALL,
29 out of 48 (60%) responded to a single agent, imatinib. However,
A phase II study in AP patients enrolled 235 patients. Some hema- the duration of response was relatively short, with a median estimated
tologic response was seen in 82% of patients, with 34% of patients time to disease progression of only 2 months.