Page 1255 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1255
Chapter 68 The Polycythemias 1101
activities, including direct effects on malignant cells, enhancement of
antitumor immune responses, induction of proapoptotic genes,
inhibition of angiogenesis, and promotion of the cycling of dormant
malignant stem cells. Because of the recent development of “targeted”
therapies, the use of IFN has been dramatically reduced over the past
decade. The increasing awareness of the numerous mutations beyond
those involving JAK2 suggest, however, that such an approach using
target-specific agents is not universally effective. This awareness
provided the rationale for the use of an agent such as IFN-α that
might be especially useful for the treatment of a disease in which
multiple genetic, epigenetic, and environmental factors contribute to
its origin and progression. IFN-α promotes apoptosis of a variety of
tumor cell types. The induction of apoptosis by IFN-α involves the
activation of a number of IFN-stimulated genes that mediate this
response. Gene expression studies have identified more than 15 IFN-
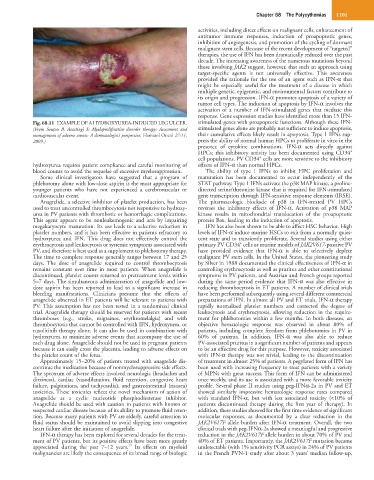
Fig. 68.11 EXAMPLE OF A HYDROXYUREA-INDUCED LEG ULCER. stimulated genes with proapoptotic functions. Although these IFN-
(From Soutou B, Aractingi S: Myeloproliferative disorder therapy: Assessment and stimulated genes alone are probably not sufficient to induce apoptosis,
management of adverse events: A dermatologist’s perspective. Hematol Oncol 27:11, their cumulative effects likely result in apoptosis. Type 1 IFNs sup-
2009.) press the ability of normal human HPCs to proliferate in vitro in the
presence of cytokine combinations. IFN-α acts directly against
+
HPCs; this inhibitory activity has been documented using CD34
+
cell populations. PV CD34 cells are more sensitive to the inhibitory
hydroxyurea requires patient compliance and careful monitoring of effects of IFN-α than normal HPCs.
blood counts to avoid the sequelae of excessive myelosuppression. The ability of type 1 IFNs to inhibit HPC proliferation and
Some clinical investigators have suggested that a program of maturation has been documented to occur independently of the
phlebotomy alone with low-dose aspirin is the most appropriate for STAT pathway. Type 1 IFNs activate the p38 MAP kinase, a proline-
younger patients who have not experienced a cerebrovascular or directed serine/threonine kinase that is required for IFN-stimulated
cardiovascular event. gene transcription through IFN-sensitive response elements (IRSE).
Anagrelide, a selective inhibitor of platelet production, has been The pharmacologic blockade of p38 in IFN-treated PV HPCs
used to treat uncontrolled thrombocytosis not responsive to hydroxy- reverses the inhibitory effects of IFN-α. Activation of p38 MAP
urea in PV patients with thrombotic or hemorrhagic complications. kinase results in mitochondrial translocation of the proapoptotic
This agent appears to be nonleukemogenic and acts by impairing protein Bax, leading to the induction of apoptosis.
megakaryocyte maturation. Its use leads to a selective reduction in IFN has also been shown to be able to affect HSC behavior. High
platelet numbers, and it has been effective in patients refractory to levels of IFN-α induce murine HSCs to exit from a normally quies-
hydroxyurea and IFN. This drug does not effectively control the cent state and to transiently proliferate. Several studies using either
+
erythrocytosis and leukocytosis or systemic symptoms associated with primary PV CD34 cells or murine models of JAK2V617-positive PV
PV, and therefore is best used as a supplement to phlebotomy therapy. have provided evidence that IFN-α is able to selectively deplete
The time to complete response generally ranges between 17 and 25 malignant PV stem cells. In the United States, the pioneering study
days. The dose of anagrelide required to control thrombocytosis by Silver in 1988 documented the clinical effectiveness of IFN-α in
remains constant over time in most patients. When anagrelide is controlling erythrocytosis as well as pruritus and other constitutional
discontinued, platelet counts returned to pretreatment levels within symptoms in PV patients, and Austrian and French groups reported
5–7 days. The simultaneous administration of anagrelide and low- during the same period evidence that IFN-α was also effective in
dose aspirin has been reported to lead to a significant increase in reducing thrombocytosis in ET patients. A number of clinical trials
bleeding manifestations. Clinicians presume that the effects of have been performed subsequently using several different commercial
anagrelide observed in ET patients will be relevant to patients with preparations of IFN. In almost all PV and ET trials, IFN-α therapy
PV. This assumption has not been tested in a randomized clinical rapidly normalized platelet numbers and corrected the degree of
trial. Anagrelide therapy should be reserved for patients with recent leukocytosis and erythrocytosis, allowing reduction in the require-
thromboses (e.g., stroke, migraines, erythromelalgia) and with ment for phlebotomies within a few months. In both diseases, an
thrombocytosis that cannot be controlled with IFN, hydroxyurea, or objective hematologic response was observed in about 80% of
ruxolitinib therapy alone. It can also be used in combination with patients, including complete freedom from phlebotomies in PV in
hydroxyurea to minimize adverse events that accompany the use of 60% of patients. In addition, IFN-α was also able to reduce
each drug alone. Anagrelide should not be used in pregnant patients PV-associated pruritus in a significant number of patients and appears
because it can easily cross the placenta, leading to adverse effects on to be an effective drug for this purpose. However, toxicity associated
the platelet count of the fetus. with IFN-α therapy was not trivial, leading to the discontinuation
Approximately 15–20% of patients treated with anagrelide dis- of treatment in almost 25% of patients. A pegylated form of IFN has
continue the medication because of nonmyelosuppressive side effects. been used with increasing frequency to treat patients with a variety
The spectrum of adverse effects involved neurologic (headaches and of MPNs with great success. This form of IFN can be administered
dizziness), cardiac (vasodilatation, fluid retention, congestive heart once weekly, and its use is associated with a more favorable toxicity
failure, palpitations, and tachycardia), and gastrointestinal (nausea) profile. Several phase II studies using peg-IFNα-2a in PV and ET
toxicities. These toxicities reflect the novel mechanism of action of showed similarly impressive hematologic response rates compared
anagrelide as a cyclic nucleotide phosphodiesterase inhibitor. with standard IFN-α, but with less associated toxicity (<10% of
Anagrelide should be used with caution in patients with known or patients discontinued therapy during the first year of therapy). In
suspected cardiac disease because of its ability to promote fluid reten- addition, these studies showed for the first time evidence of significant
tion. Because many patients with PV are elderly, careful attention to molecular responses, as documented by a clear reduction in the
fluid status should be maintained to avoid slipping into congestive JAK2V617F allele burden after IFN-α treatment. Overall, the two
heart failure after the initiation of anagrelide. clinical trials with peg-IFNα-2a showed a meaningful and progressive
IFN-α therapy has been explored for several decades for the treat- reduction in the JAK2V617F allele burden in about 70% of PV and
ment of PV patients, but its positive effects have been more greatly 40% of ET patients. Importantly, the JAK2V617F mutation became
29
appreciated during the past 7–12 years. Its effects on myeloid undetectable (with 1% sensitivity PCR assays) in 24% of PV patients
malignancies are likely the consequence of its broad range of biologic in the French PVN-1 study after about 3 years’ median follow-up,