Page 1262 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1262
1108 Part VII Hematologic Malignancies
Cell membrane
CALR MUT + + + CALR MUT + + + MPL
2 3
ER
- - -
KDEL
JAK–STAT
CALR WT − − − activation 4
- - - KDEL
KDEL
CALR WT
Oncogenic
1 transformation 5
p.L367fs ∗ 46
CALR MUT
Nucleus
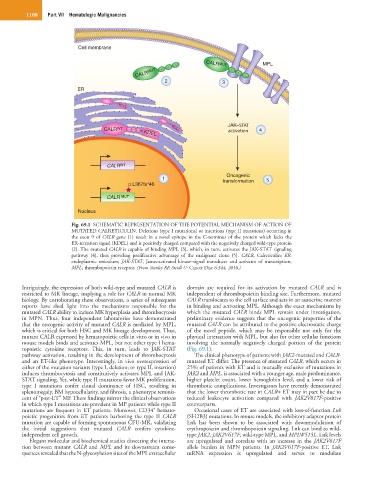
Fig. 69.1 SCHEMATIC REPRESENTATION OF THE POTENTIAL MECHANISM OF ACTION OF
MUTATED CALRETICULIN. Deletions (type I mutations) or insertions (type II mutations) occurring in
the exon 9 of CALR gene (1) result in a novel epitope in the C-terminus of the protein which lacks the
ER-retention signal (KDEL) and is positively charged compared with the negatively charged wild-type protein
(2). The mutated CALR is capable of binding MPL (3), which, in turn, activates the JAK-STAT signaling
pathway (4), thus providing proliferative advantage of the malignant clone (5). CALR, Calreticulin; ER,
endoplasmic reticulum; JAK-STAT, Janus-activated kinase–signal transducer and activator of transcription;
MPL, thrombopoietin receptor. (From Stanley RF, Steidl U: Cancer Disc 6:344, 2016.)
Intriguingly, the expression of both wild-type and mutated CALR is domain are required for its activation by mutated CALR and is
restricted to MK lineage, implying a role for CALR in normal MK independent of thrombopoietin binding site. Furthermore, mutated
biology. By corroborating these observations, a series of subsequent CALR translocates to the cell surface and acts in an autocrine manner
reports have shed light into the mechanisms responsible for the in binding and activating MPL. Although the exact mechanisms by
mutated CALR ability to induce MK hyperplasia and thrombocytosis which the mutated CALR binds MPL remain under investigation,
in MPN. Thus, four independent laboratories have demonstrated preliminary evidence suggests that the oncogenic properties of the
that the oncogenic activity of mutated CALR is mediated by MPL, mutated CALR can be attributed to the positive electrostatic charge
which is critical for both HSC and MK lineage development. Thus, of the novel peptide, which may be responsible not only for the
mutant CALR expressed by hematopoietic cells in vitro or in vivo in physical interaction with MPL, but also for other cellular functions
mouse models binds and activates MPL, but not other type I hema- involving the normally negatively charged portion of the protein
topoietic cytokine receptors. This, in turn, leads to JAK-STAT (Fig. 69.1).
pathway activation, resulting in the development of thrombocytosis The clinical phenotype of patients with JAK2-mutated and CALR-
and an ET-like phenotype. Interestingly, in vivo overexpression of mutated ET differ. The presence of mutated CALR, which occurs in
either of the mutation variants (type I, deletion; or type II, insertion) 25% of patients with ET and is mutually exclusive of mutations in
induces thrombocytosis and constitutively activates MPL and JAK- JAK2 and MPL, is associated with a younger age, male predominance,
STAT signaling. Yet, while type II mutations favor MK proliferation, higher platelet count, lower hemoglobin level, and a lower risk of
type I mutations confer clonal dominance of HSC, resulting in thrombotic complications. Investigators have recently demonstrated
splenomegaly, BM hypocellularity, and fibrosis, a phenotype reminis- that the lower thrombotic rate in CALR+ ET may in part be due to
cent of “post-ET” MF. These findings mirror the clinical observations reduced leukocyte activation compared with JAK2V617F-positive
in which type I mutations are prevalent in MF patients while type II counterparts.
+
mutations are frequent in ET patients. Moreover, CD34 hemato- Occasional cases of ET are associated with loss-of-function Lnk
poietic progenitors from ET patients harboring the type II CALR (SH2B3) mutations. In mouse models, the inhibitory adaptor protein
mutation are capable of forming spontaneous CFU-MK, validating Lnk has been shown to be associated with downmodulation of
the initial suggestions that mutated CALR confers cytokine- erythropoietin and thrombopoietin signaling. Lnk can bind to wild-
independent cell growth. type JAK2, JAK2V617F, wild-type MPL, and MPLW515L. Lnk levels
Elegant molecular and biochemical studies dissecting the interac- are upregulated and correlate with an increase in the JAK2V617F
tion between mutant CALR and MPL and its downstream conse- allele burden in MPN patients. In JAK2V617F-positive ET, Lnk
quences revealed that the N-glycosylation sites of the MPL extracellular mRNA expression is upregulated and serves to modulate