Page 1263 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1263
Chapter 69 Essential Thrombocythemia 1109
JAK2V617F-mediated cell regulation. Thrombopoietin-mediated The incidence of thrombotic events in ET patients ranges from
signaling regulates Lnk expression at both the mRNA and protein 1.5% to 6.6% per patient-year. The thrombotic events in ET are
levels. Furthermore, acquired Lnk mutations have been observed in largely arterial, but venous thromboses also occur not infrequently.
less than 1% of ET. The inactivating Lnk mutations in ET patients JAK2V617F-positive ET patients compared with patients with
result in JAK-STAT activation, leading to high levels of STAT3 and patients with a CALR mutation or those who are triple negative have
STAT5 activation. a higher risk of developing thrombotic events, but a dose-dependent
Recently, Cabagnois and coworkers used whole-exome sequencing correlation between allele burden and the development of clinical
and next-generation sequencing targeting JAK2 and MPL with the symptoms has not been demonstrated. The age-related differences in
intent of detecting additional mutations in triple-negative ET the frequency of these events have been attributed to the coexistence
patients. They found several signaling mutations including of vascular disease in older patients. Many investigators have struggled
JAK2V617F at very low allele frequency, as well as additional muta- to identify ET patients who are at the greatest risk of developing
tions such as: LNK mutation, MPL-S505N, MPL-W515R, and thrombotic episodes. Recently, a new thrombotic scoring system has
1
MPL-S204P. MPL-S204P and MPL-Y591N were shown to be weak been developed by investigators on both sides of the Atlantic. In
gain-of-function mutants increasing MPL signaling and conferring the latest iteration of this risk stratification schema four categories
either thrombopoietin hypersensitivity or independence to expressing were delineated: very low risk (no thrombosis history, age ≤60 years,
cells, but with a low efficiency. These data demonstrate that some and JAK2 unmutated); low risk (no thrombosis history, age ≤60
clonal, noncanonical MPL gain-of-function mutations are associated years, and JAK2 mutated); intermediate risk (no thrombosis history,
with triple-negative cases of ET. In the triple-negative patients in age >60 years, and JAK2 unmutated) and high risk (thrombosis
whom these mutations were not detected, a number had clonal while history, age >60 years, and JAK2 mutation). How to personalize
the others had polyclonal hematopoiesis, suggesting that certain therapy based upon such a schema remains problematic and to date
patients with triple-negative ET cannot be classified as having an untested. 2
MPN and should be evaluated for inherited forms of thrombocytosis Some progress has been made in defining the relationship between
that will be discussed later. platelet numbers and the risks for thrombotic and hemorrhagic
With the development of whole-exome sequencing, mutations in events in ET. In a randomized trial of patients at a high risk of
epigenetic regulators (such as TET2, DNMT3A, ASXL1, EZH2, and developing a thrombotic event (>60 years of age, a history of a
isocitrate dehydrogenase [IDH]1/IDH2) and in spliceosome compo- thrombotic episode, or both), the reduction of platelet numbers
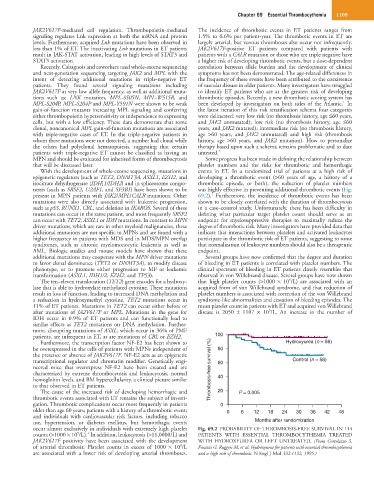
nents (such as SRSF2, U2AF1, and SF3B1) have been shown to be was highly effective in preventing additional thrombotic events (Fig.
5
present in MPN patients with JAK2/MPL/CALR mutations. Other 69.2). Furthermore, the incidence of thrombotic events has been
mutations were also directly associated with leukemic progression, shown to be closely correlated with the duration of thrombocytosis
such as p53, RUNX1, CBL, and deletion in IKAROS. Several of these in a case–control study. Unfortunately, there has been difficulty in
mutations can occur in the same patient, and most frequently SRSF2 defining what particular target platelet count should serve as an
can occur with TET2, ASXL1 or IDH mutations. In contrast to MPN endpoint for myelosuppressive therapies to maximally reduce the
driver mutations, which are rare in other myeloid malignancies, these degree of thrombotic risk. Many investigators have provided data that
additional mutations are not specific to MPNs and are found with a indicate that interactions between platelets and activated leukocytes
higher frequency in patients with MDS and in MDS/MPN overlap participate in the thrombotic risk of ET patients, suggesting to some
syndromes, such as chronic myelomonocytic leukemia as well as that normalization of leukocyte numbers should also be a therapeutic
AML. Biologic studies and mouse models have shown that these endpoint.
additional mutations may cooperate with the MPN driver mutations Several groups have now confirmed that the degree and duration
to favor clonal dominance (TET2 or DNMT3A), to modify disease of bleeding in ET patients is correlated with platelet numbers. The
phenotype, or to promote either progression to MF or leukemic clinical spectrum of bleeding in ET patients closely resembles that
transformation (ASXL1, IDH1/2, EZH2, and TP53). observed in von Willebrand disease. Several groups have now shown
9
The ten–eleven translocation (TET2) gene encodes for a hydroxy- that high platelet counts (>1000 × 10 /L) are associated with an
lase that is able to hydroxylate methylated cytosine. These mutations acquired form of von Willebrand syndrome, and that reduction of
result in loss of function, leading to increased DNA methylation and platelet numbers is associated with correction of the von Willebrand
a reduction in hydroxymethyl cytosine. TET2 mutations occur in syndrome-like abnormalities and cessation of bleeding episodes. The
11% of ET patients. Mutations in TET2 can occur either before or mean platelet count in patients with ET and acquired von Willebrand
9
after mutations of JA2V617F or MPL. Mutations in the gene for disease is 2050 ± 1107 × 10 /L. An increase in the number of
IDH occur in 0.9% of ET patients and can functionally lead to
similar effects as TET2 mutations on DNA methylation. Further-
more, disrupting mutations of ASXL, which occur in 36% of PMF
patients, are infrequent in ET, as are mutations of CBL or EZH2. 100
Furthermore, the transcription factor NF-E2 has been shown to Hydroxyurea (n = 56)
be overexpressed in the cells of patients with MPNs independent of 80
the presence or absence of JAK2V617F. NF-E2 acts as an epigenetic
transcriptional regulator and chromatin modifier. Genetically engi- 60 Control (n = 58)
neered mice that overexpress NF-E2 have been created and are Thrombosis-free survival (%)
characterized by extreme thrombocytosis and leukocytosis, normal 40
hemoglobin levels, and BM hypercellularity, a clinical picture similar
to that observed in ET patients.
The cause of the increased risk of developing hemorrhagic and 20 P = 0.005
thrombotic events associated with ET remains the subject of investi-
gation. Thrombotic complications occur most frequently in patients 0
older than age 60 years; patients with a history of a thrombotic event; 0 6 12 18 24 30 36 42 48
and individuals with cardiovascular risk factors, including tobacco
use, hypertension, or diabetes mellitus, but hemorrhagic events Months after randomization
occur almost exclusively in individuals with extremely high platelet Fig. 69.2 PROBABILITY OF THROMBOSIS-FREE SURVIVAL IN 114
9
4
counts (>1000 × 10 /L). In addition, leukocytosis (>11,0000/L) and PATIENTS WITH ESSENTIAL THROMBOCYTHEMIA TREATED
JAK2V617F positivity have been associated with the development WITH HYDROXYUREA OR LEFT UNTREATED. (From Cortelazzo S,
9
of arterial thrombosis. Platelet counts in excess of 1000 × 10 /L Finazzi G, Ruggeri M, et al: Hydroxyurea for patients with essential thrombocythemia
are associated with a lower risk of developing arterial thromboses. and a high risk of thrombosis. N Engl J Med 332:1132, 1995.)