Page 1267 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1267
Chapter 69 Essential Thrombocythemia 1113
A B C
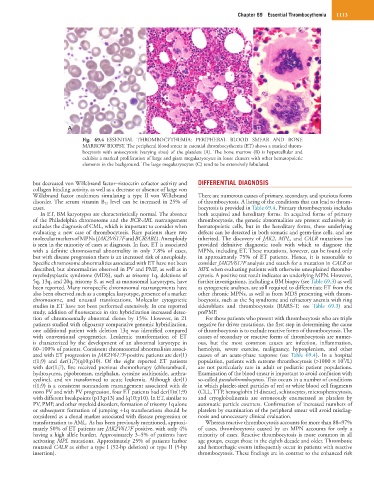
Fig. 69.4 ESSENTIAL THROMBOCYTHEMIA: PERIPHERAL BLOOD SMEAR AND BONE
MARROW BIOPSY. The peripheral blood smear in essential thrombocythemia (ET) shows a marked throm-
bocytosis with anisocytosis (varying sizes) of the platelets (A). The bone marrow (B) is hypercellular and
exhibits a marked proliferation of large and giant megakaryocytes in loose clusters with other hematopoietic
elements in the background. The large megakaryocytes (C) tend to be extensively lobulated.
but decreased von Willebrand factor–ristocetin cofactor activity and DIFFERENTIAL DIAGNOSIS
collagen binding activity, as well as a decrease or absence of large von
Willebrand factor multimers simulating a type II von Willebrand There are numerous causes of primary, secondary, and spurious forms
disorder. The serum vitamin B 12 level can be increased in 25% of of thrombocytosis. A listing of the conditions that can lead to throm-
cases. bocytosis is provided in Table 69.4. Primary thrombocytosis includes
In ET, BM karyotypes are characteristically normal. The absence both acquired and hereditary forms. In acquired forms of primary
of the Philadelphia chromosome and the BCR-ABL rearrangement thrombocytosis, the genetic abnormalities are present exclusively in
excludes the diagnosis of CML, which is important to consider when hematopoietic cells, but in the hereditary forms, these underlying
evaluating a new case of thrombocytosis. Rare patients share two defects can be detected in both somatic and germ-line cells, and are
molecular markers of MPNs (JAK2V617F and BCR/ABL). Aneuploidy inherited. The discovery of JAK2, MPL, and CALR mutations has
is seen in the minority of cases at diagnosis. In fact, ET is associated provided definitive diagnostic tools with which to diagnose the
with a definite chromosomal abnormality in only 7.8% of cases, MPNs, including ET. These mutations, however, can be found only
but with disease progression there is an increased risk of aneuploidy. in approximately 75% of ET patients. Hence, it is reasonable to
Specific chromosome abnormalities associated with ET have not been consider JAK2V617F analysis and search for a mutation in CALR or
described, but abnormalities observed in PV and PMF, as well as in MPL when evaluating patients with otherwise unexplained thrombo-
myelodysplastic syndrome (MDS), such as trisomy 1q, deletions of cytosis. A positive test result indicates an underlying MPN. However,
5q, 13q, and 20q, trisomy 8, as well as monosomal karyotypes, have further investigations, including a BM biopsy (see Table 69.3) as well
been reported. Many nonspecific chromosomal rearrangements have as cytogenetic analyses, are still required to differentiate ET from the
also been observed such as a complex karyotype, presence of a marker other chronic MPNs, as well as from MDS presenting with throm-
chromosome, and unusual translocations. Molecular cytogenetic bocytosis, such as the 5q syndrome and refractory anemia with ring
studies in ET have not been performed extensively. In one reported sideroblasts and thrombocytosis (RARS-T; see Table 69.3) and
study, addition of fluorescence in situ hybridization increased detec- prePMF.
tion of chromosomally abnormal clones by 15%. However, in 21 For those patients who present with thrombocytosis who are triple
patients studied with oligoarray comparative genomic hybridization, negative for driver mutations, the first step in determining the cause
one additional patient with deletion 13q was identified compared of thrombocytosis is to exclude reactive forms of thrombocytosis. The
with conventional cytogenetics. Leukemic transformation of ET causes of secondary or reactive forms of thrombocytosis are numer-
is characterized by the development of an abnormal karyotype in ous, but the most common causes are infection, inflammation,
60–100% of patients. Consistent chromosomal abnormalities associ- hemolysis, severe exercise, malignancy, hyposplenism, and other
ated with ET progression in JAK2V617F-positive patients are der(1) causes of an acute-phase response (see Table 69.4). In a hospital
9
t(1;9) and der(1;7)(q10;p10). Of the eight reported ET patients population, patients with extreme thrombocytosis (>1000 × 10 /L)
with der(1;7), five received previous chemotherapy (chlorambucil, are not particularly rare in adult or pediatric patient populations.
hydroxyurea, pipobroman, melphalan, cytosine arabinoside, anthra- Examination of the blood smear is important to avoid confusion with
cycline), and six transformed to acute leukemia. Although der(1) so-called pseudothrombocytosis. This occurs in a number of conditions
t(1;9) is a consistent nonrandom rearrangement associated with de in which platelet-sized particles of red or white blood cell fragments
novo PV and with PV progression, four ET patients had der(1)t(1;9) (CLL, TTP, hemoglobin H disease), schistocytes, microspherocytosis,
with different breakpoints (p13;p13) and (q10;p10). In ET, similar to and cryoglobulinemia are erroneously enumerated as platelets by
PV, PMF, and other myeloid disorders, formation of trisomy 1q alone automatic particle counters. Confirmation of increased numbers of
or subsequent formation of jumping +1q translocations should be platelets by examination of the peripheral smear will avoid misdiag-
considered as a clonal marker associated with disease progression or nosis and unnecessary clinical evaluation.
transformation to AML. As has been previously mentioned, approxi- Whereas reactive thrombocytosis accounts for more than 88–97%
mately 50% of ET patients are JAK2V617F positive, with only 4% of cases, thrombocytosis caused by an MPN accounts for only a
having a high allele burden. Approximately 3–5% of patients have minority of cases. Reactive thrombocytosis is more common in all
activating MPL mutations. Approximately 25% of patients harbor age groups, except those in the eighth decade and older. Thrombotic
mutated CALR as either a type I (52-bp deletion) or type II (5-bp and hemorrhagic events infrequently occur in patients with reactive
insertion). thrombocytosis. These findings are in contrast to the enhanced risk