Page 1275 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1275
Chapter 69 Essential Thrombocythemia 1121
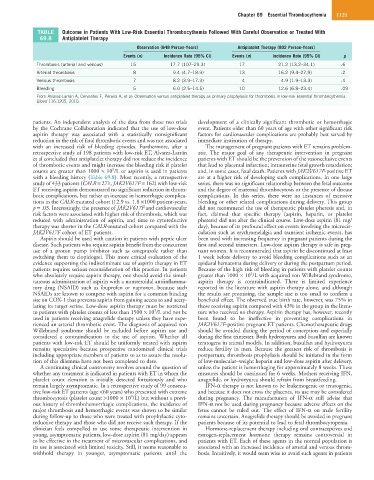
TABLE Outcome in Patients With Low-Risk Essential Thrombocythemia Followed With Careful Observation or Treated With
69.8 Antiplatelet Therapy
Observation (848 Person-Years) Antiplatelet Therapy (802 Person-Years)
Events (n) Incidence Rate (95% CI) Events (n) Incidence Rate (95% CI) p
Thrombosis (arterial and venous) 15 17.7 (107–29.3) 17 21.2 (13.2–34.1) .6
Arterial thrombosis 8 9.4 (4.7–18.9) 13 16.2 (9.4–27.9) .2
Venous thrombosis 7 8.2 (3.9–17.3) 4 4.9 (1.9–13.3) .4
Bleeding 5 6.0 (2.5–14.5) 10 12.6 (6.8–23.4) .09
From Alvarez-Larrán A, Cervantes F, Pereira A, et al: Observation versus antiplatelet therapy as primary prophylaxis for thrombosis in low-risk essential thrombocythemia.
Blood 116:1205, 2010.
patients. An independent analysis of the data from these two trials development of a clinically significant thrombotic or hemorrhagic
by the Cochrane Collaboration indicated that the use of low-dose event. Patients older than 60 years of age with other significant risk
aspirin therapy was associated with a statistically nonsignificant factors for cardiovascular complications are probably best served by
reduction in the risk of fatal thrombotic events and was not associated immediate institution of therapy.
with an increased risk of bleeding episodes. Furthermore, after a The management of pregnant patients with ET remains problem-
retrospective study of 198 patients with low-risk ET, Alvarez-Larrán atic. The major goal of any therapeutic intervention in pregnant
et al concluded that antiplatelet therapy did not reduce the incidence patients with ET should be the prevention of the vasoocclusive events
of thrombotic events and might increase the bleeding risk if platelet that lead to placental infarction; intrauterine fetal growth retardation;
9
counts are greater than 1000 × 10 /L or aspirin is used in patients and, in some cases, fetal death. Patients with JAK2V617F-positive ET
with a bleeding history (Table 69.8). Most recently, a retrospective are at a higher risk of developing such complications. In one large
study of 433 patients (CALR = 271, JAK2V617F = 162) with low-risk series, there was no significant relationship between the fetal outcome
ET receiving aspirin demonstrated no significant reduction in throm- and the degree of maternal thrombocytosis or the presence of disease
botic complications, but rather an increase in hemorrhagic complica- complications. In this series, there were no instances of excessive
tions in the CALR-mutated cohort (12.9 vs. 1.8 ×1000 patient-years, bleeding or other related complications during delivery. This group
p = .03. Interestingly, the presence of JAK2V617F and cardiovascular did not recommend the use of therapeutic platelet pheresis and, in
risk factors were associated with higher risk of thrombosis, which was fact, claimed that specific therapy (aspirin, heparin, or platelet
reduced with administration of aspirin, and time to cytoreductive pheresis) did not alter the clinical course. Low-dose aspirin (81 mg/
therapy was shorter in the CALR-mutated cohort compared with the day), because of its profound effect on events involving the microcir-
JAK2V617F cohort of ET patients. culation such as erythromelalgia and transient ischemic events, has
Aspirin should be used with caution in patients with peptic ulcer been used with increasing frequency in pregnant patients during the
disease. Such patients who require aspirin benefit from the concurrent first and second trimesters. Low-dose aspirin therapy is safe in preg-
use of a proton pump inhibitor such as omeprazole rather than nant women. It is recommended that aspirin be discontinued at least
switching them to clopidogrel. This more critical evaluation of the 1 week before delivery to avoid bleeding complications such as an
evidence supporting the indiscriminate use of aspirin therapy in ET epidural hematoma during delivery or during the postpartum period.
patients requires serious reconsideration of this practice. In patients Because of the high risk of bleeding in patients with platelet counts
9
who absolutely require aspirin therapy, one should avoid the simul- greater than 1000 × 10 /L with acquired von Willebrand syndrome,
taneous administration of aspirin with a nonsteroidal antiinflamma- aspirin therapy is contraindicated. There is limited experience
tory drug (NSAID) such as ibuprofen or naproxen, because such reported in the literature with aspirin therapy alone, and although
NSAIDs are known to compete with aspirin for a common binding the results are promising, the sample size is too small to confirm a
site on COX-1 that prevents aspirin from gaining access to and acety- beneficial effect. The observed true birth rate, however, was 75% in
lating its target serine. Low-dose aspirin therapy must be restricted those receiving aspirin compared with 43% in the group in the litera-
9
to patients with platelet counts of less than 1500 × 10 /L and not be ture who received no therapy. Aspirin therapy has, however, recently
used in patients receiving anagrelide therapy unless they have expe- been found to be ineffective in preventing complications in
rienced an arterial thrombotic event. The diagnosis of acquired von JAK2V617F-positive pregnant ET patients. Chemotherapeutic drugs
Willebrand syndrome should be excluded before aspirin use and should be avoided during the period of conception and especially
considered a contraindication to the use of aspirin. Whether all during the first trimester. Both hydroxyurea and busulfan are known
patients with low-risk ET should be uniformly treated with aspirin teratogens in animal models. In addition, busulfan and hydroxyurea
remains speculative because prospective randomized clinical trials reduce fertility in men. Because the greatest risk of thrombosis is
including appropriate numbers of patients so as to assure the resolu- postpartum, thrombosis prophylaxis should be initiated in the form
tion of this dilemma have not been completed to date. of low-molecular–weight heparin and low-dose aspirin after delivery,
A continuing clinical controversy revolves around the question of unless the patient is hemorrhaging for approximately 8 weeks. These
whether any treatment is indicated in patients with ET in whom the measures should be continued for 6 weeks. Mothers receiving IFN,
platelet count elevation is initially detected fortuitously and who anagrelide, or hydroxyurea should refrain from breastfeeding.
remain largely asymptomatic. In a retrospective study of 99 consecu- IFN-α therapy is not known to be leukemogenic or teratogenic,
tive low-risk ET patients (age <60 years) who presented with extreme and because it does not cross the placenta, its use may be considered
9
thrombocytosis (platelet count >1000 × 10 /L) but without a previ- during pregnancy. The manufacturers of IFN-α still advise that
ous history of thrombohemorrhagic complications, the incidence of IFN-α not be used during pregnancy because adverse effects on the
major thrombosis and hemorrhagic events was shown to be similar fetus cannot be ruled out. The effect of IFN-α on male fertility
during follow-up to those who were treated with prophylactic cyto- remains uncertain. Anagrelide therapy should be avoided in pregnant
reductive therapy and those who did not receive such therapy. If the patients because of its potential to lead to fetal thrombocytopenia.
clinician feels compelled to use some therapeutic intervention in Hormone-replacement therapy including oral contraceptives and
young, asymptomatic patients, low-dose aspirin (81 mg/day) appears estrogen-replacement hormone therapy remains controversial in
to be effective in the treatment of microvascular complications, and patients with ET. Each of these agents in the normal population is
its use is associated with limited toxicity. Still, it seems reasonable to associated with an increased incidence of arterial and venous throm-
withhold therapy in younger, asymptomatic patients until the bosis. Intuitively, it would seem wise to avoid such agents in patients