Page 1273 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1273
Chapter 69 Essential Thrombocythemia 1119
An alternative to hydroxyurea is therapy with anagrelide. Data on 100
the use of anagrelide in ET suggest that it is nonleukemogenic. When Hydroxyurea + aspirin
considering the risk-to-benefit ratio, one can conclude that hydroxy-
urea therapy is a first-line therapy for ET patients at a high risk of 90 89%
developing an additional thrombosis, including those older than 60
years of age or with a history of a thrombotic episode or with signifi- Anagrelide + aspirin
cant other cardiovascular risk factors. Such nonleukemogenic drugs Event-free survival (%) 80 84%
as IFN-α, anagrelide, or pegylated IFN appear to be good choices in
symptomatic patients younger than 40 years of age.
Anagrelide is a member of the imidazo(2,1-b)quinazolin-2-1 series
of compounds. When studied in humans, it was noted that anagrelide 70
in small doses produced thrombocytopenia. The drug acts primarily P = .03
by reducing megakaryocyte size and ploidy, and decreasing mega- 0
karyocyte proliferation. Anagrelide therefore appears to lower platelet 0 1 2 3 4 5
counts, primarily by interfering with the development of megakaryo-
cytes. Anagrelide suppresses megakaryocytopoiesis by selectively Time after trial entry (yr)
reducing the expression levels of GATA-1, FLI-1, NFE-2, and FOG-1 No. at risk
in cells belonging to the megakaryocytic lineage. The effects of Hydroxyurea 404 388 298 204 129 57
anagrelide do not involve thrombopoietin-mediated signal transduc- plus aspirin
tion events. GATA-1 and FOG-1 play critical roles in megakaryocytic Anagrelide 405 379 272 190 119 52
differentiation. Anagrelide in low doses is effective in lowering the plus aspirin
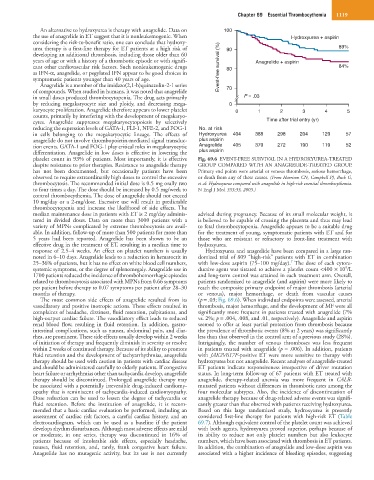
platelet count in 93% of patients. Most importantly, it is effective Fig. 69.6 EVENT-FREE SURVIVAL IN A HYDROXYUREA-TREATED
despite resistance to prior therapies. Resistance to anagrelide therapy GROUP COMPARED WITH AN ANAGRELIDE-TREATED GROUP.
has not been documented, but occasionally patients have been Primary end points were arterial or venous thrombosis, serious hemorrhage,
observed to require extraordinarily high doses to control the excessive or death from any of these causes. (From Harrison CN, Campbell PJ, Buck G,
thrombocytosis. The recommended initial dose is 0.5 mg orally two et al: Hydroxyurea compared with anagrelide in high-risk essential thrombocythemia.
to four times a day. The dose should be increased by 0.5 mg/week to N Engl J Med 353:33, 2005.)
control thrombocythemia. The dose of anagrelide should not exceed
10 mg/day or a 2-mg/dose. Excessive use will result in predictable
thrombocytopenia and increase the likelihood of side effects. The
median maintenance dose in patients with ET is 2 mg/day adminis- advised during pregnancy. Because of its small molecular weight, it
tered in divided doses. Data on more than 3000 patients with a is believed to be capable of crossing the placenta and thus may lead
variety of MPNs complicated by extreme thrombocytosis are avail- to fetal thrombocytopenia. Anagrelide appears to be a suitable drug
able. In addition, follow-up of more than 500 patients for more than for the treatment of young, symptomatic patients with ET and for
5 years had been reported. Anagrelide has been shown to be an those who are resistant or refractory to front-line treatment with
effective drug in the treatment of ET, resulting in a median time to hydroxyurea.
response of 2.5–4 weeks. An effect on platelet numbers is usually Hydroxyurea and anagrelide have been compared in a large ran-
noted in 6–10 days. Anagrelide leads to a reduction in hematocrit in domized trial of 809 “high-risk” patients with ET in combination
7
25–36% of patients, but it has no effect on white blood cell numbers, with low-dose aspirin (75–100 mg/day). The dose of each cytore-
9
systemic symptoms, or the degree of splenomegaly. Anagrelide use in ductive agent was titrated to achieve a platelet count <400 × 10 /L
1700 patients reduced the incidence of thrombohemorrhagic episodes and long-term control was attained in each treatment arm. Overall,
related to thrombocytosis associated with MPNs from 0.66 symptoms patients randomized to anagrelide (and aspirin) were more likely to
per patient before therapy to 0.07 symptoms per patient after 28–30 reach the composite primary endpoint of major thrombosis (arterial
months of therapy. or venous), major hemorrhage, or death from vascular causes
The most common side effects of anagrelide resulted from its (p = .03; Fig. 69.6). When individual endpoints were assessed, arterial
vasodilatory and positive inotropic actions. These effects resulted in thrombosis, major hemorrhage, and the development of MF were all
complaints of headache, dizziness, fluid retention, palpitations, and significantly more frequent in patients treated with anagrelide (7%
high-output cardiac failure. The vasodilatory effect leads to reduced vs. 2%; p = .004, .008, and .01, respectively). Anagrelide and aspirin
renal blood flow, resulting in fluid retention. In addition, gastro- seemed to offer at least partial protection from thrombosis because
intestinal complications, such as nausea, abdominal pain, and diar- the prevalence of thrombotic events (8% at 2 years) was significantly
rhea, are prominent. These side effects usually develop within 2 weeks less than that observed in the control arm of a previous study (28%).
of initiation of therapy and frequently diminish in severity or resolve Intriguingly, the number of venous thromboses was less frequent
within 2 weeks of continued therapy. Because of its ability to promote in patients treated with anagrelide (p = .006). In addition, patients
fluid retention and the development of tachyarrhythmias, anagrelide with JAK2V617F-positive ET were more sensitive to therapy with
therapy should be used with caution in patients with cardiac disease hydroxyurea but not anagrelide. Recent analyses of anagrelide-treated
and should be administered carefully to elderly patients. If congestive ET patients indicate responsiveness irrespective of driver mutation
heart failure or arrhythmias other than tachycardia develop, anagrelide status. In long-term follow-up of 67 patients with ET treated with
therapy should be discontinued. Prolonged anagrelide therapy may anagrelide, therapy-related anemia was more frequent in CALR-
be associated with a potentially irreversible drug-induced cardiomy- mutated patients without differences in thrombotic rates among the
opathy that is reminiscent of tachycardia-induced cardiomyopathy. four molecular subtypes. Also, the incidence of discontinuation of
Dose reduction can be used to lessen the degree of tachycardia or anagrelide therapy because of drug-related adverse events was signifi-
fluid retention. Before the institution of anagrelide, it is recom- cantly greater than that observed with patients receiving hydroxyurea.
mended that a basic cardiac evaluation be performed, including an Based on this large randomized study, hydroxyurea is presently
assessment of cardiac risk factors, a careful cardiac history, and an considered first-line therapy for patients with high-risk ET (Table
electrocardiogram, which can be used as a baseline if the patient 69.7). Although equivalent control of the platelet count was achieved
develops rhythm disturbances. Although most adverse effects are mild with both agents, hydroxyurea proved superior, perhaps because of
or moderate, in one series, therapy was discontinued in 16% of its ability to reduce not only platelet numbers but also leukocyte
patients because of intolerable side effects, especially headache, numbers, which have been associated with thrombosis in ET patients.
nausea, fluid retention, and, rarely, frank congestive heart failure. In addition, the combination of anagrelide and low-dose aspirin was
Anagrelide has no mutagenic activity, but its use is not currently associated with a higher incidence of bleeding episodes, suggesting