Page 1282 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1282
1128 Part VII Hematologic Malignancies
expression of the mutant C-terminus alone nor expression of CALR mutations throughout the 11 exons of this gene result in com promised
lacking the C-terminus leads to cytokine-independent growth, sug- catalytic activity of an α-ketoglutarate-dependent enzyme responsible
gesting that the novel C-terminus is necessary (but not sufficient) for for oxidation of 5-methylcytosine (5mC) to 5-hydroxymethylcytosine
transformation. They found that the oncogenic activity of mutant (5hmC) in DNA. Low levels of 5hmC result in a hypermethylation
CALR is not encoded within a specific sequence or domain of the phenotype at CpG sites in various DNA promoter regions. TET2
−
+
hi
mutant C-terminus, but that the positive electrostatic charge of the mRNA has been found to be highly expressed in Lin , Sca-1 , c-Kit
mutant C-terminus was critical for its transforming capacity. Muta- murine multipotent progenitor cells isolated from the BM and
genizing all 18 lysine/arginine residues (positively charged) within the thymus. This expression pattern was maintained in myeloid progeni-
C-terminus to a neutral glycine residue abrogated CALR-del52 tor cells but low in mature granulocytes. Moreover, in patient samples,
transformation activity. In contrast, mutagenizing the 18 nonlysine/ when compared with normal samples, low 5hmC levels correlated
arginine residues within the C-terminus to glycine did not affect with a significant decrease in DNA hypermethylation, supporting a
transforming activity, a remarkable finding considering that, in this role of TET2 loss of function leading to DNA hypermethylation. It
mutant, 50% of the amino acids have been modified, Using coim- is currently believed that TET2 mutations result in loss of function
munoprecipitation assays, Elf et al found that mutant CALR, but not and lead to the accumulation of 5mC in DNA, promoting DNA
WT CALR, physically interacted with MPL, and that neither the hypermethylation that may inhibit cells from differentiating beyond
mutant C-terminus alone nor mutant CALR lacking the C-terminus a HSC-like state. In vitro studies reveal that TET2 deficiency restrains
can bind to MPL. This suggests that the tertiary structure of mutant hematopoietic cells from normal differentiation patterns and skews
CALR is required for binding to MPL. differentiation in favor of the monocyte/macrophage lineage with
Approximately 10% of PMF patients lack any of the three driver transgenic animals, with knockout of TET2 having a phenotype
mutations. However, recently Milosevic-Feenstra and coworkers resembling chronic myelomonocytic leukemia. Somatic, recurrent
identified noncanonical MPL mutations by whole-exon sequencing TET2 mutations have been reported in normal elderly individuals
in eight out of 70 (11.4%) and JAK2 mutations in five out of 57 with acquired clonal hematopoiesis. Alterations in TET2 that include
(8.8%) patients with triple-negative ET and PMF. All mutations were base substitutions, out-of-frame insertions or deletions, and splice site
heterozygous. The mutations in MPL and JAK2 were mutually mutations have been shown to occur in 15–20% of PMF patients.
exclusive. Evidence for clonal disease was lacking in 50% of these Such mutations are more common in older patients. In families with
triple-negative cases and the presence of germ-line mutations indi- multiple family members having an MPN, all TET2 mutations were
cated that some of these individuals likely had a hereditary rather almost universally acquired, and their incidence was similar to that
than an acquired MPN-like disorder. Based upon these findings, of patients with sporadic PMF. Clonal analysis studies in MPN
sequencing of all coding exons of MPL and JAK2 during the diag- patient samples failed to define a consistent temporal sequence of
nostic work-up of ET and PMF patients who are triple negative is acquisition of TET2 mutations with respect to JAK2 mutations and
recommended. can occur late in the progression of MPNs. These two genetic events
A growing list of additional mutations have been identified in appear to occur independently.TET2 mutational status in PMF
PMF patients over the past several years, providing further insight patients does not appear to provide a new prognostic marker. TET2
6
into the complex molecular pathology of this disease. Many of these mutations can be found in PMF patients with and without the
+
mutations impact the epigenome and can coexist in PMF CD34 JAK2V617F mutation, and do not influence rate of thrombosis,
cells. None of the genetic or epigenetic lesions identified thus far are leukemic transformation, or OS.
specific to PMF, clearly aid in prognostication, or appear to be Isocitrate dehydrogenase 1 and 2 (IDH1/2), located on chromo-
+
disease-initiating events. Table 70.2 lists the molecular lesions that some 2q33.3 and 15q26.1, respectively, encode NADP -dependent
have been characterized in patients with PMF. enzymes that catalyze the oxidative decarboxylation of isocitrate
Translocation ten-eleven oncogene family member 2 (TET2), to α-ketoglutarate. Mutant IDH forms preferentially transform
located on chromosome 4q24, has been identified in many myeloid α-ketoglutarate to 2-hydroxyglutarate and appear to promote
malignancies at a frequency of approximately 15%. Acquired somatic tumorigenesis by inducing hypoxia-inducible factor 1α (HIF-1α).
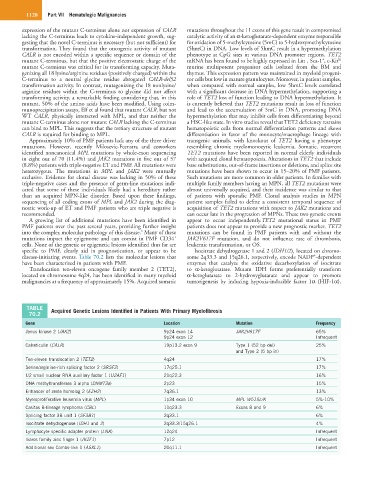
TABLE Acquired Genetic Lesions Identified in Patients With Primary Myelofibrosis
70.2
Gene Location Mutation Frequency
Janus kinase 2 (JAK2) 9p24 exon 14 JAK2V617F 65%
9p24 exon 12 Infrequent
Calreticulin (CALR) 19p13.2 exon 9 Type 1 (52 bp del) 25%
and Type 2 (5 bp in)
Ten-eleven translocation 2 (TET2) 4q24 17%
Serine/arginine-rich splicing factor 2 (SRSF2) 17q25.1 17%
U2 small nuclear RNA auxillary factor 1 (U2AF1) 21q22.3 16%
DNA methyltransferase 3 alpha (DNMT3a) 2p23 15%
Enhancer of zeste homolog 2 (EZH2) 7q36.1 13%
Myeloproliferative leukemia virus (MPL) 1p34 exon 10 MPL W515L/K 5%-10%
Casitas B-lineage lymphoma (CBL) 11q23.3 Exons 8 and 9 6%
Splicing factor 3B unit 1 (SF3B1) 2q33.1 6%
Isocitrate dehydrogenase (IDH1 and 2) 2q33.3/15q26.1 4%
Lymphocyte specific adapter protein (LNK) 12q24 Infrequent
Ikaros family zinc finger 1 (IKZF1) 7p12 Infrequent
Additional sex Combs-like 1 (ASXL1) 20q11.1 Infrequent