Page 1297 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1297
Chapter 70 Primary Myelofibrosis 1143
thrombocytopenia, which occurred in approximately 30% of patients of patients with long-term maintenance therapy enjoy sustained relief
but resolved with discontinuation of therapy. In several patients with of symptoms. Hematologic toxicity often necessitates cessation of
del(5)(q31)-associated PMF or post-PV MF, lenalidomide therapy therapy. Inhibition of the activated JAK-STAT pathway is an attrac-
was associated with reduction of the numbers of cells with a marker tive therapeutic target in PMF and has led to the development of oral
chromosome as assayed by FISH and reduction of the JAK2V617F small-molecule inhibitors. Ruxolitinib, a selective JAK1 and JAK2
burden. Lenalidomide therapy in combination with prednisone tyrosine kinase inhibitor, was approved in 2011 for the treatment of
therapy in PMF has been evaluated in a phase II trial within the patients with MF; this is the first drug approved by the United States
Eastern Cooperative Oncology Group and appears to be modestly Food and Drug Administration (US FDA) where the indication for
active and myelosuppressive. Forty two MF patients with anemia use is intermediate- or high-risk MF. The phase I/II study determined
were treated with lenalidomide at 10 mg/day in combination with a the optimal starting dose of 15 mg twice daily, and that thrombocy-
3-month prednisone taper. Grade 3 and higher myelotoxicity was topenia and worsening anemia were dose-limiting toxicities. A dose-
noted in 88% of patients, and a response rate of 23% by IWG-MRT dependent suppression of phospho-STAT3 was seen in both WT and
was obtained (19% clinical improvement in anemia or 10% clinical mutated JAK2 patients treated with ruxolitinib, demonstrating that
improvement in spleen size). Whether either of these immunomodu- drug activity does not discriminate between mutated and WT JAK2
latory drugs (IMiDs) is superior to the other is the subject of specula- in its inhibitory activity. Fifty two percent of treated patients achieved
tion, which would require a large randomized clinical trial. The a greater than 50% reduction in splenomegaly for greater than 12
mechanism by which these agents achieve these clinical responses also months, which was irrespective of their JAK2 mutational status. The
remains of larger speculation. debilitating symptoms associated with MF were dramatically
Because IMiDs as a class have shown activity in MF and their use improved, and this was correlated with reduction in inflammatory
is often undermined by neurotoxicity or myelosuppression, recent cytokines believed to be mediated through inhibition of JAK1 signal-
interest in the structurally related but more potent pomalidomide in ing. This compound was then evaluated in two randomized phase III
the treatment of MF-associated anemia has led to the completion of trials, COMFORT 1 (United States, Canada, and Australia), in
several early-phase studies and a randomized phase III study. The first which ruxolitinib therapy was compared with a placebo control, and
study to evaluate the response of pomalidomide in MF was a phase COMFORT 2 trial (Europe), in which ruxolitinib therapy was
II randomized, double-blind, placebo-controlled adaptive design compared with the best available therapy. 12,13 The results of each of
study with four treatment arms. This study reported an overall these trials are remarkably similar, and each trial was adequately
response in anemia of 24% (10 patients), and 15 of these patients powered. Patients with intermediate-2 or high-risk MF as assessed
achieved transfusion independence that was durable at 7.5 months. using the IPSS were eligible regardless of JAK2 mutational status. All
9
Pomalidomide at 0.5 mg/day with or without a prednisone taper was patients had platelet counts over 100 × 10 /L and had a spleen pal-
found to have better anemia responses with less toxicity compared pated at least 5 cm below the left costal margin. In COMFORT 1,
with pomalidomide at 2 mg/day with or without prednisone. Grade the primary endpoint of 35% or greater reduction in spleen volume
3/4 myelosuppression and neurotoxicity were not frequent adverse was met at 24 weeks in 41.9% of the ruxolitinib-treated cohort and
events. A single-center phase II study of low-dose pomalidomide was only 0.7% in the placebo group. This response was maintained 48
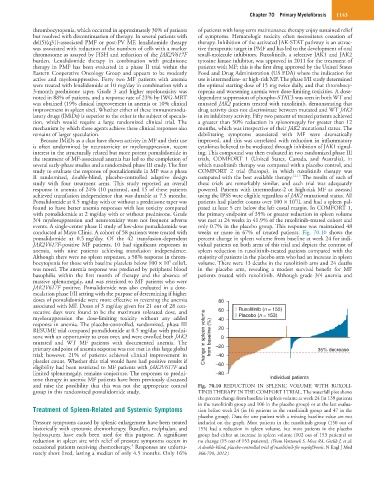
conducted at Mayo Clinic. A cohort of 58 patients were treated with weeks or more in 67% of treated patients. Fig. 70.10 shows the
pomalidomide at 0.5 mg/day. Of the 42 transfusion-dependent percent change in spleen volume from baseline at week 24 for indi-
JAK2V617F-positive MF patients, 10 had significant responses in vidual patients on both arms of this trial and depicts the contrast of
anemia, with nine patients achieving transfusion independence. spleen reduction in ruxolitinib-treated patients compared with the
Although there were no spleen responses, a 58% response in throm- majority of patients in the placebo arm who had an increase in spleen
9
bocytopenia for those with baseline platelets below 100 × 10 cells/L volume. There were 13 deaths in the ruxolitinib arm and 24 deaths
was noted. The anemia response was predicted by peripheral blood in the placebo arm, revealing a modest survival benefit for MF
basophilia within the first month of therapy and the absence of patients treated with ruxolitinib. Although grade 3/4 anemia and
massive splenomegaly, and was restricted to MF patients who were
JAK2V617F positive. Pomalidomide was also evaluated in a dose-
escalation phase I/II setting with the purpose of determining if higher
doses of pomalidomide were more effective in reversing the anemia 80
associated with MF. Doses of 3 mg/day given for 21 out of 28 con- Ruxolitinib (n = 155)
secutive days were found to be the maximum tolerated dose, and 60 Placebo (n = 153)
myelosuppression the dose-limiting toxicity without any added 40
response in anemia. The placebo-controlled, randomized, phase III
RESUME trial compared pomalidomide at 0.5 mg/day with predni- 20
sone with an opportunity to cross over, and were enrolled both JAK2 Change in spleen volume from baseline (%) 0
mutated and WT MF patients with documented anemia. The
primary endpoint of anemia response was not met in this large global –20 35% decrease
trial; however, 21% of patients achieved clinical improvement in –40
platelet count. Whether this trial would have had positive results if
eligibility had been restricted to MF patients with JAK2V617F and –60
limited splenomegaly, remains conjecture. The responses to predni- –80
sone therapy in anemic MF patients have been previously discussed Individual patients
and raise the possibility that this was not the appropriate control Fig. 70.10 REDUCTION IN SPLENIC VOLUME WITH RUXOLI-
group in this randomized pomalidomide study. TINIB THERAPY IN THE COMFORT I TRIAL. The waterfall plot shows
the percent change from baseline in spleen volume at week 24 (in 139 patients
in the ruxolitinib group and 106 in the placebo group) or at the last evalua-
Treatment of Spleen-Related and Systemic Symptoms tion before week 24 (in 16 patients in the ruxolitinib group and 47 in the
placebo group). Data for one patient with a missing baseline value are not
Pressure symptoms caused by splenic enlargement have been treated included on the graph. Most patients in the ruxolitinib group (150 out of
historically with cytotoxic chemotherapy. Busulfan, melphalan, and 155) had a reduction in spleen volume, but most patients in the placebo
hydroxyurea have each been used for this purpose. A significant group had either an increase in spleen volume (102 out of 153 patients) or
reduction in spleen size with relief of pressure symptoms occurs in no change (15 out of 153 patients). (From Verstovsek S, Mesa RA, Gotlib J, et al:
4
occasional patients receiving chemotherapy. Responses are unfortu- A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N Engl J Med
nately short lived, lasting a median of only 4.5 months. Only 16% 366:799, 2012.)