Page 1292 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1292
1138 Part VII Hematologic Malignancies
because of the different modalities of treatment that can be success- in CML occurs in two distinct patterns, one in which patients present
fully used for hairy cell leukemia. with CML and significant associated BM fibrosis, and a second in
BM fibrosis can occur in patients with other MPNs, especially PV which the MF develops late in the course of the CML. The MF in
and CML, and less frequently with ET. In CML, progressive BM the latter group appears at a mean of 36 months after the diagnosis
fibrosis may herald the onset of accelerated disease or blast crisis. MF of CML, is associated with a mean survival time of 4.9 months from
the detection of MF, and therefore represents an ominous prognostic
sign.
TABLE World Health Organization Criteria for Primary Post-PV MF occurs in 5–15% of patients with PV. This transition
70.5 Myelofibrosis a occurs, on average, 10 years after the initial diagnosis of PV is made,
Major Criteria but in individual cases it may appear after shorter or longer intervals.
b
1. Presence of megakaryocyte proliferation and atypia, usually PMF is clinically indistinguishable from post-PV MF except for the
accompanied by either reticulin or collagen fibrosis, or, in the previous history of erythrocytosis in the latter group. Of patients with
absence of significant reticulin fibrosis, the megakaryocyte changes post-PV MF, 25–50% develop leukemia, and 70% are dead within
must be accompanied by an increased bone marrow cellularity 3 years of this transition. Post-PV MF represents a transitional
characterized by granulocytic proliferation and often decreased myeloproliferative syndrome with relatively grave prognostic implica-
erythropoiesis (i.e., prefibrotic cellular-phase disease) tions. MF has also been reported after ET. These investigators claimed
d
c
e
2. Not meeting WHO criteria for PV, CML, MDS, or other myeloid that these patients did not represent individuals with prefibrotic
neoplasm stages of PMF but rather evolution of patients with true ET. They
3. Demonstration of JAK2617V>F or other clonal marker (e.g., estimated the probability of developing such a complication to be
MPL515W>L/K), or in the absence of a clonal marker, no evidence 3% 5 years after diagnosis, 8% at 10 years, and 15% at 15 years, and
of bone marrow fibrosis caused by underlying inflammatory or other considered this evolution to BM fibrosis a major long-term complica-
neoplastic diseases f tion of ET.
Minor Criteria Acute panmyelosis with myelofibrosis (APMF) represents a clini-
1. Leukoerythroblastosis g cal entity distinct from PMF (see Fig. 70.6). This disorder has also
2. Increase in serum lactate dehydrogenase level g been termed acute MF, acute myelosclerosis, acute megakaryocytic MF,
3. Anemia g and acute myelodysplasia with MF. APMF is exceedingly rare and
4. Palpable splenomegaly g corresponds to less than 1% of the cases of AML. Patients character-
a Diagnosis requires meeting all three major criteria and two minor criteria. istically present with pancytopenia, fever, absence of clinically signifi-
b Small-to-large megakaryocytes with an aberrant nuclear-to-cytoplasmic ratio cant splenomegaly, minimal or absent teardrop poikilocytosis, and
and hyperchromatic, bulbous, or irregularly folded nuclei and dense clustering. fibrotic BM. The BM is characterized by the appearance of immature
c Requires the failure of iron-replacement therapy to increase hemoglobin level myeloid cells and blast cells, which frequently express megakaryocytic
to the polycythemia vera range in the presence of decreased serum ferritin. phenotypic properties. Survival ranges from 1 to 9 months after
Exclusion of polycythemia vera is based on hemoglobin and hematocrit levels. diagnosis. Its distinction from PMF is important because aggressive
Red blood cell mass measurement is not required.
d Requires the absence of BCR-ABL. chemotherapy and possibly SCT are the treatments of choice. Up to
e Requires the absence of dyserythropoiesis and dysgranulopoiesis. 12% of patients who present with MF have been reported to have
f Secondary to infection, autoimmune disorder or other chronic inflammatory an underlying autoimmune disorder such as SLE, although in the
condition, hairy cell leukemia or other lymphoid neoplasm, metastatic authors’ clinical practice, this is an extraordinary rare event. Primary
malignancy, or toxic (chronic) myelopathies. It should be noted that patients
with conditions associated with reactive myelofibrosis are not immune to autoimmune MF (primary AIMF) likely represents a distinct clini-
primary myelofibrosis, and the diagnosis should be considered in such cases if copathologic syndrome unrelated to other well-defined autoimmune
other criteria are met. disorders. Eight diagnostic criteria for AIMF, including grade 3 or 4
g Degree of abnormality could be borderline or marked. reticulin fibrosis in the BM, lack of clustered or atypical megakaryo-
CML, Chronic myeloid leukemia; MDS, myelodysplastic syndrome; PV,
polycythemia vera; WHO, World Health Organization. cytes, lack of dysplasia or eosinophilia or basophilia, lymphoid
Data from Tefferi A, Thiele J, Orazi A, et al: Proposals and rationale for revision infiltration of the BM, lack of osteosclerosis, absent or mild spleno-
of the World Health Organization diagnostic criteria for polycythemia vera, megaly, presence of autoantibodies, and absence of disorders associ-
essential thrombocythemia, and primary myelofibrosis: Recommendations from ated with MF, have been outlined. Autoimmune MF occurs
an ad hoc international expert panel. Blood 110:1092, 2007.
predominantly in females with a broad clinical spectrum. Patients
A B
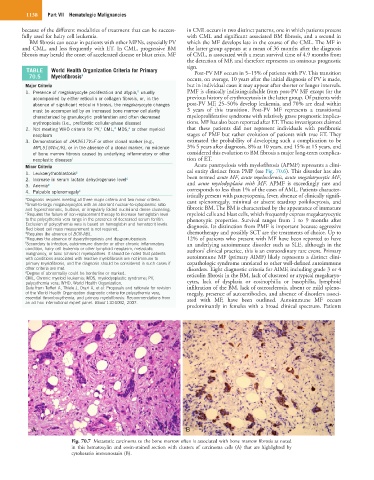
Fig. 70.7 Metastatic carcinoma to the bone marrow often is associated with bone marrow fibrosis as noted
in this hematoxylin and eosin-stained section with clusters of carcinoma cells (A) that are highlighted by
cytokeratin immunostain (B).