Page 1402 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1402
1248 Part VII Hematologic Malignancies
C
D
A B E F G
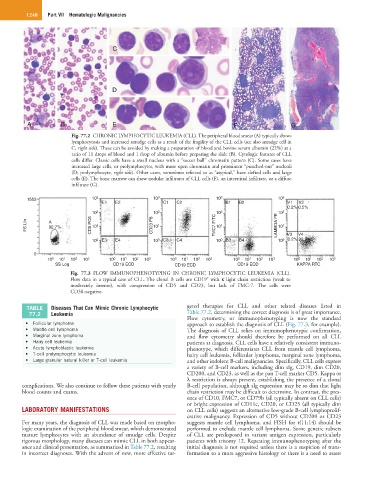
Fig. 77.2 CHRONIC LYMPHOCYTIC LEUKEMIA (CLL). The peripheral blood smear (A) typically shows
lymphocytosis and increased smudge cells as a result of the fragility of the CLL cells (see also smudge cell in
C, right side). These can be avoided by making a preparation of blood and bovine serum albumin (22%) at a
ratio of 11 drops of blood and 1 drop of albumin before preparing the slide (B). Cytologic features of CLL
cells differ. Classic cells have a small nucleus with a “soccer ball” chromatin pattern (C). Some cases have
increased large cells, or prolymphocytes, with more open chromatin and prominent “punched-out” nucleoli
(D; prolymphocyte, right side). Other cases, sometimes referred to as “atypical,” have clefted cells and large
cells (E). The bone marrow can show nodular infiltrates of CLL cells (F), an interstitial infiltrate, or a diffuse
infiltrate (G).
1553 10 3 10 3 10 3 10 3
E1 E2 C1 C2 B1 B2 V1 V2
0.2% 0.5%
10 2 10 2 2 2
FS LIn A CD5 PC6 10 1 CD23 P8 10 1 FMC7 FITC 10 1 LAMBDA PE 10 1
10
10
92.7%
V3 V4
10 0 E3 E4 10 0 C3 C4 10 0 B3 B4 10 0 0.2% 99.2%
0
10 0 10 1 10 2 10 3 10 0 10 1 10 2 10 3 10 0 10 1 10 2 10 3 10 0 10 1 10 2 10 3 10 0 10 1 10 2 10 3
SS Log CD19 ECD CD19 ECD CD19 ECD KAPPA RTC
Fig. 77.3 FLOW IMMUNOPHENOTYPING IN CHRONIC LYMPHOCYTIC LEUKEMIA (CLL).
+
Flow data in a typical case of CLL. The clonal B cells are CD19 with κ light chain restriction (weak to
moderately intense), with coexpression of CD5 and CD23, but lack of FMC-7. The cells were
CD38-negative.
TABLE Diseases That Can Mimic Chronic Lymphocytic geted therapies for CLL and other related diseases listed in
77.2 Leukemia Table 77.2, determining the correct diagnosis is of great importance.
Flow cytometry, or immunophenotyping is now the standard
• Follicular lymphoma approach to establish the diagnosis of CLL (Fig. 77.3, for example).
• Mantle cell lymphoma The diagnosis of CLL relies on immunophenotypic confirmation,
• Marginal zone lymphoma and flow cytometry should therefore be performed on all CLL
• Hairy cell leukemia patients at diagnosis. CLL cells have a relatively consistent immuno-
• Acute lymphoblastic leukemia phenotype, which differentiates CLL from mantle cell lymphoma,
• T-cell prolymphocytic leukemia hairy cell leukemia, follicular lymphoma, marginal zone lymphoma,
• Large granular natural killer or T-cell leukemia and other indolent B-cell malignancies. Specifically, CLL cells express
a variety of B-cell markers, including dim sIg, CD19, dim CD20,
CD200, and CD23, as well as the pan T-cell marker CD5. Kappa or
λ restriction is always present, establishing the presence of a clonal
complications. We also continue to follow these patients with yearly B-cell population, although sIg expression may be so dim that light
blood counts and exams. chain restriction may be difficult to determine. In contrast, the pres-
ence of CD10, FMC7, or CD79b (all typically absent on CLL cells)
or bright expression of CD11c, CD20, or CD25 (all typically dim
LABORATORY MANIFESTATIONS on CLL cells) suggests an alternative low-grade B-cell lymphoprolif-
erative malignancy. Expression of CD5 without CD200 or CD23
For many years, the diagnosis of CLL was made based on morpho- suggests mantle cell lymphoma, and FISH for t(11;14) should be
logic examination of the peripheral blood smear, which demonstrated performed to exclude mantle cell lymphoma. Some genetic subsets
mature lymphocytes with an abundance of smudge cells. Despite of CLL are predisposed to variant antigen expression, particularly
rigorous morphology, many diseases can mimic CLL in both appear- patients with trisomy 12. Repeating immunophenotyping after the
ance and clinical presentation, as summarized in Table 77.2, resulting initial diagnosis is not required unless there is a suspicion of trans-
in incorrect diagnoses. With the advent of new, more effective tar- formation to a more aggressive histology or there is a need to assess