Page 1403 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1403
Chapter 77 Chronic Lymphocytic Leukemia 1249
A B C D E
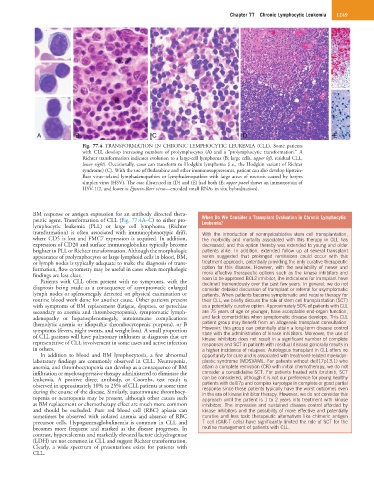
Fig. 77.4 TRANSFORMATION IN CHRONIC LYMPHOCYTIC LEUKEMIA (CLL). Some patients
with CLL develop increasing numbers of prolymphocytes (A) and a “prolymphocytic transformation.” A
Richter transformation indicates evolution to a large-cell lymphoma (B; large cells, upper left, residual CLL,
lower right). Occasionally, cases can transform to Hodgkin lymphoma (i.e., the Hodgkin variant of Richter
syndrome) (C). With the use of fludarabine and other immunosuppressants, patient can also develop Epstein-
Barr virus–related lymphadenopathies or lymphadenopathies with large areas of necrosis caused by herpes
simplex virus (HSV). The case illustrated in (D) and (E) had both (E; upper panel shows an immunostain of
HSV-1/2, and lower is Epstein-Barr virus—encoded small RNAs in situ hybridization).
BM response or antigen expression for an antibody directed thera-
peutic agent. Transformation of CLL (Fig. 77.4A–C) to either pro- When Do We Consider a Transplant Evaluation in Chronic Lymphocytic
Leukemia?
lymphocytic leukemia (PLL) or large cell lymphoma (Richter
transformation) is often associated with immunophenotypic drift, With the introduction of nonmyeloablative stem cell transplantation,
where CD5 is lost and FMC7 expression is acquired. In addition, the morbidity and mortality associated with this therapy in CLL has
expression of CD20 and surface immunoglobulins typically become decreased, and this option thereby was extended to young and older
brighter in PLL or Richter transformation. Although the morphologic patients alike. In addition, extended follow up at several transplant
appearance of prolymphocytes or large lymphoid cells in blood, BM, series suggested that prolonged remissions could occur with this
or lymph nodes is typically adequate to make the diagnosis of trans- treatment approach, potentially providing the only curative therapeutic
formation, flow cytometry may be useful in cases when morphologic option for this disease. However, with the availability of newer and
findings are less clear. more effective therapeutic options such as the kinase inhibitors and
soon to be approved BCL2 inhibitor, the indications for transplant have
Patients with CLL often present with no symptoms, with the declined tremendously over the past few years. In general, we do not
diagnosis being made as a consequence of asymptomatic enlarged consider detailed discussion of transplant or referral for asymptomatic
lymph nodes or splenomegaly detected on physical examination or patients. When patients become symptomatic and require therapy for
routine blood work done for another cause. Other patients present their CLL, we briefly discuss the role of stem cell transplantation (SCT)
with symptoms of BM replacement (fatigue, dyspnea, or petechiae as a potentially curative option. Approximately 50% of patients with CLL
secondary to anemia and thrombocytopenia), symptomatic lymph- are 75 years of age or younger, have acceptable end-organ function,
adenopathy or hepatosplenomegaly, autoimmune complications and lack comorbidities when symptomatic disease develops. This CLL
(hemolytic anemia or idiopathic thrombocytopenic purpura), or B patient group may benefit from an allogeneic transplant consultation.
symptoms (fevers, night sweats, and weight loss). A small proportion However, this group can potentially attain a long-term disease control
state with the administration of kinase inhibitors. Moreover, the use of
of CLL patients will have pulmonary infiltrates at diagnosis that are kinase inhibitors does not result in a significant number of complete
representative of CLL involvement in some cases and active infection responses and SCT in patients with residual disease generally results in
in others. a higher incidence of relapses. Autologous transplant in CLL offers no
In addition to blood and BM lymphocytosis, a few abnormal opportunity for cure and is associated with treatment-related myelodys-
laboratory findings are commonly observed in CLL. Neutropenia, plastic syndrome (MDS)/AML. For patients without del(17p13.1) who
anemia, and thrombocytopenia can develop as a consequence of BM attain a complete remission (CR) with initial chemotherapy, we do not
infiltration or myelosuppressive therapy administered to eliminate the consider a consolidative SCT. For patients treated with ibrutinib, SCT
leukemia. A positive direct antibody, or Coombs, test result is can be considered, although it is not our preference for young healthy
observed in approximately 10% to 25% of CLL patients at some time patients with del17p and complex karyotype in complete or good partial
response since these patients typically have the worst outcomes even
during the course of the disease. Similarly, autoimmune thrombocy- in the era of kinase inhibitor therapy. However, we do not consider this
topenia or neutropenia may be present, although other causes such approach until the patient is 1 to 2 years into treatment with kinase
as BM replacement or chemotherapy effect are much more common inhibitors. The impressive and sustained disease control afforded by
and should be excluded. Pure red blood cell (RBC) aplasia can kinase inhibitors and the possibility of more effective and potentially
sometimes be observed with isolated anemia and absence of RBC curative and less toxic therapeutic alternatives like chimeric antigen
precursor cells. Hypogammaglobulinemia is common in CLL and T cell (CAR-T cells) have significantly limited the role of SCT for the
becomes more frequent and marked as the disease progresses. In routine management of patients with CLL.
contrast, hypercalcemia and markedly elevated lactate dehydrogenase
(LDH) are not common in CLL and suggest Richter transformation.
Clearly, a wide spectrum of presentations exists for patients with
CLL.