Page 155 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 155
Chapter 10 Stem Cell Model of Hematologic Diseases 113
Functional Evaluation of Cell-of-Origin in Vivo
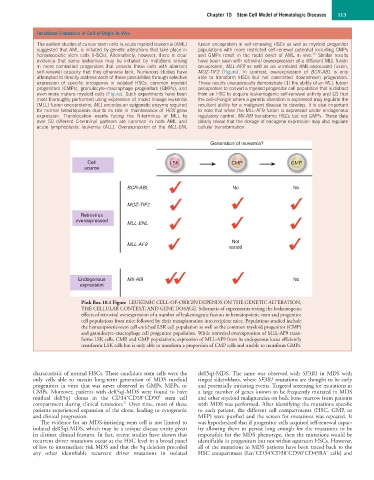
The earliest studies of cancer stem cells in acute myeloid leukemia (AML) fusion oncoprotein in self-renewing HSCs as well as myeloid progenitor
suggested that AML is initiated by genetic alterations that take place in populations with more restricted self-renewal potential including CMPs
10
hematopoietic stem cells (HSCs). Alternatively, however, there is clear and GMPs result in the rapid onset of AML in vivo. Similar results
evidence that some leukemias may be initiated by mutations arising have been seen with retroviral overexpression of a different MLL fusion
in more committed progenitors that provide these cells with aberrant oncoprotein, MLL-AF9 as well as an unrelated AML-associated fusion,
self-renewal capacity that they otherwise lack. Numerous studies have MOZ-TIF2 (Figure). In contrast, overexpression of BCR-ABL is only
attempted to directly address each of these possibilities through selective able to transform HSCs but not committed downstream progenitors.
expression of specific oncogenes in isolated HSCs, common myeloid These results unequivocally demonstrate (1) the ability of an MLL fusion
progenitors (CMPs), granulocyte–macrophage progenitors (GMPs), and oncoprotein to convert a myeloid progenitor cell population that is distinct
even more mature myeloid cells (Figure). Such experiments have been from an HSC to acquire leukemogenic self-renewal activity and (2) that
most thoroughly performed using expression of mixed lineage leukemia the cell-of-origin where a genetic alteration is expressed may regulate the
(MLL) fusion oncoproteins. MLL encodes an epigenetic enzyme required resultant ability for a malignant disease to develop. It is also important
for normal hematopoiesis due to its role in maintenance of HOX gene to note that when the MLL-AF9 fusion is expressed under endogenous
expression. Translocation events fusing the N-terminus of MLL to regulatory control, Mll-Af9 transforms HSCs but not GMPs. These data
over 50 different C-terminal partners are common in both AML and clearly reveal that the dosage of oncogene expression may also regulate
acute lymphoblastic leukemia (ALL). Overexpression of the MLL-ENL cellular transformation.
Generation of leukemia?
Cell LSK CMP GMP
source
BCR-ABL No No
MOZ-TIF2
Retrovirus
overexpressed MLL-ENL
Not
MLL-AF9
tested
Endogenous MII-Af9 No
expression
Pink Box 10.1 Figure LEUKEMIC CELL-OF-ORIGIN DEPENDS ON THE GENETIC ALTERATION,
THE CELLULAR CONTEXT, AND GENE DOSAGE. Schematic of experiments testing the leukemogenic
effects of retroviral overexpression of a number of leukemogenic fusions in hematopoietic stem and progenitor
cell populations from mice followed by their transplantation into recipient mice. Populations studied include
the hematopoietic-stem cell enriched LSK cell population as well as the common myeloid progenitor (CMP)
and granulocyte–macrophage cell progenitor population. While retroviral overexpression of MLL-AF9 trans-
forms LSK cells, CMP, and GMP populations, expression of MLL-AF9 from its endogenous locus efficiently
transforms LSK cells but is only able to transform a proportion of CMP cells and unable to transform GMPs.
characteristic of normal HSCs. These candidate stem cells were the del(5q)-MDS. The same was observed with SF3B1 in MDS with
only cells able to sustain long-term generation of MDS myeloid ringed sideroblasts, where SF3B1 mutations are thought to be early
progenitors in vitro that was never observed in GMPs, MEPs, or and potentially initiating events. Targeted screening for mutations in
CMPs. Moreover, patients with del(5q)-MDS were found to have a large number of genes known to be frequently mutated in MDS
+
−
+
residual del(5q) clones in the CD34 CD38 CD90 stem cell and other myeloid malignancies on bulk bone marrow from patients
21
compartment during clinical remission. Over time, most of these with MDS was performed. After identifying the mutations specific
patients experienced expansion of the clone, leading to cytogenetic to each patient, the different cell compartments (HSC, GMP, or
and clinical progression. MEP) were purified and the screen for mutations was repeated. It
The evidence for an MDS-initiating stem cell is not limited to was hypothesized that if progenitor cells acquired self-renewal capac-
isolated del(5q)-MDS, which may be a unique disease entity given ity allowing them to persist long enough for the mutations to be
its distinct clinical features. In fact, recent studies have shown that responsible for the MDS phenotype, then the mutations would be
recurrent driver mutations occur at the HSC level in a broad panel identifiable in progenitors but not within upstream HSCs. However,
of low to intermediate risk MDS and that the 5q deletion preceded all of the mutations in MDS patients have been traced back to the
+
−
−
−
+
any other identifiable recurrent driver mutations in isolated HSC compartment (Lin CD34 CD38 CD90 CD45RA cells) and