Page 1555 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1555
1382 Part VII Hematologic Malignancies
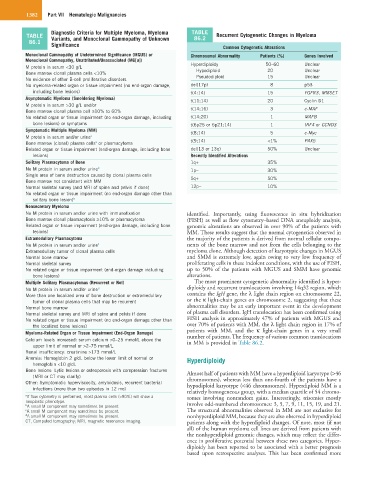
Diagnostic Criteria for Multiple Myeloma, Myeloma TABLE
TABLE Variants, and Monoclonal Gammopathy of Unknown 86.2 Recurrent Cytogenetic Changes in Myeloma
86.1 Significance
Common Cytogenetic Alterations
Monoclonal Gammopathy of Undetermined Significance (MGUS) or Chromosomal Abnormality Patients (%) Genes Involved
Monoclonal Gammopathy, Unattributed/Unassociated (MG[u])
M protein in serum <30 g/L Hyperdiploidy 50–60 Unclear
Unclear
20
Hypodiploid
Bone marrow clonal plasma cells <10% Pseudodiploid 15 Unclear
No evidence of other B-cell proliferative disorders
No myeloma-related organ or tissue impairment (no end-organ damage, del(17p) 8 p53
including bone lesions) t(4:14) 15 FGFR3, MMSET
Asymptomatic Myeloma (Smoldering Myeloma) t(11;14) 20 Cyclin D1
M protein in serum >30 g/L and/or
Bone marrow clonal plasma cell ≥10% to 60% t(14;16) 3 c-MAF
No related organ or tissue impairment (no end-organ damage, including t(14;20) 1 MAFB
bone lesions) or symptoms t(6p25 or 6p21;14) 1 IRF4 or CCND3
Symptomatic Multiple Myeloma (MM) t(8;14) 5 c-Myc
M protein in serum and/or urine a
Bone marrow (clonal) plasma cells or plasmacytoma t(9;14) <1% PAX5
a
Related organ or tissue impairment (end-organ damage, including bone del(13 or 13q) 50% Unclear
lesions) Recently Identified Alterations
Solitary Plasmacytoma of Bone 1q+ 35%
No M protein in serum and/or urine b 1p− 30%
Single area of bone destruction caused by clonal plasma cells 5q+ 50%
Bone marrow not consistent with MM
Normal skeletal survey (and MRI of spine and pelvis if done) 12p− 10%
No related organ or tissue impairment (no end-organ damage other than
solitary bone lesion) b
Nonsecretory Myeloma
No M protein in serum and/or urine with immunofixation identified. Importantly, using fluorescence in situ hybridization
Bone marrow clonal plasmacytosis ≥10% or plasmacytoma (FISH) as well as flow cytometry–based DNA aneuploidy analysis,
Related organ or tissue impairment (end-organ damage, including bone genomic alterations are observed in over 90% of the patients with
lesions) MM. These results suggest that the normal cytogenetics observed in
Extramedullary Plasmacytoma the majority of the patients is derived from normal cellular compo-
No M protein in serum and/or urine c nents of the bone marrow and not from the cells belonging to the
Extramedullary tumor of clonal plasma cells myeloma clone. Although detection of karyotypic changes in MGUS
Normal bone marrow and SMM is extremely low, again owing to very low frequency of
Normal skeletal survey proliferating cells in these indolent conditions, with the use of FISH,
No related organ or tissue impairment (end-organ damage including up to 50% of the patients with MGUS and SMM have genomic
bone lesions) alterations.
Multiple Solitary Plasmacytomas (Recurrent or Not) The most prominent cytogenetic abnormality identified is hyper-
No M protein in serum and/or urine d diploidy and recurrent translocations involving 14q32 region, which
More than one localized area of bone destruction or extramedullary contains the IgH gene, the λ light chain region on chromosome 22,
tumor of clonal plasma cells that may be recurrent or the κ light-chain genes on chromosome 2, suggesting that these
Normal bone marrow abnormalities may be an early important event in the development
Normal skeletal survey and MRI of spine and pelvis if done of plasma cell disorders. IgH translocation has been confirmed using
No related organ or tissue impairment (no end-organ damage other than FISH analysis in approximately 47% of patients with MGUS and
the localized bone lesions) over 70% of patients with MM, the λ light chain region in 17% of
Myeloma-Related Organ or Tissue Impairment (End-Organ Damage) patients with MM, and the κ light-chain genes in a very small
number of patients. The frequency of various common translocations
Calcium levels increased: serum calcium >0–25 mmol/L above the in MM is provided in Table 86.2.
upper limit of normal or >2–75 mmol/L
Renal insufficiency: creatinine >173 mmol/L
Anemia: Hemoglobin 2 g/dL below the lower limit of normal or Hyperdiploidy
hemoglobin <10 g/dL
Bone lesions: Lytic lesions or osteoporosis with compression fractures Almost half of patients with MM have a hyperdiploid karyotype (>46
(MRI or CT may clarify) chromosomes), whereas less than one-fourth of the patients have a
Other: Symptomatic hyperviscosity, amyloidosis, recurrent bacterial hypodiploid karyotype (<46 chromosomes). Hyperdiploid MM is a
infections (more than two episodes in 12 mo) relatively homogeneous group, with a median quartile of 54 chromo-
a If flow cytometry is performed, most plasma cells (>90%) will show a somes involving nonrandom gains. Interestingly, trisomies mostly
neoplastic phenotype.
b A small M component may sometimes be present. involve odd-numbered chromosomes: 3, 5, 7, 9, 11, 15, 19, and 21.
c A small M component may sometimes be present. The structural abnormalities observed in MM are not exclusive for
d A small M component may sometimes be present. nonhyperdiploid MM, because they are also observed in hyperdiploid
CT, Computed tomography; MRI, magnetic resonance imaging. patients along with the hyperdiploid changes. Of note, most (if not
all) of the human myeloma cell lines are derived from patients with
the nonhyperdiploid genomic changes, which may reflect the differ-
ence in proliferative potential between these two categories. Hyper-
diploidy has been reported to be associated with a better prognosis
based upon retrospective analyses. This has been confirmed more