Page 1597 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1597
Chapter 87 Waldenström Macroglobulinemia/Lymphoplasmacytic Lymphoma 1423
A
B
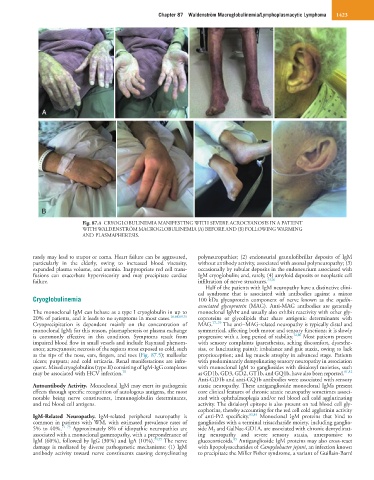
Fig. 87.4 CRYOGLOBULINEMIA MANIFESTING WITH SEVERE ACROCYANOSIS IN A PATIENT
WITH WALDENSTRÖM MACROGLOBULINEMIA (A) BEFORE AND (B) FOLLOWING WARMING
AND PLASMAPHERESIS.
rarely may lead to stupor or coma. Heart failure can be aggravated, polyneuropathies; (2) endoneurial granulofibrillar deposits of IgM
particularly in the elderly, owing to increased blood viscosity, without antibody activity, associated with axonal polyneuropathy; (3)
expanded plasma volume, and anemia. Inappropriate red cell trans- occasionally by tubular deposits in the endoneurium associated with
fusions can exacerbate hyperviscosity and may precipitate cardiac IgM cryoglobulin; and, rarely, (4) amyloid deposits or neoplastic cell
failure. infiltration of nerve structures. 73,76
Half of the patients with IgM neuropathy have a distinctive clini-
cal syndrome that is associated with antibodies against a minor
Cryoglobulinemia 100 kDa glycoprotein component of nerve known as the myelin-
associated glycoprotein (MAG). Anti-MAG antibodies are generally
The monoclonal IgM can behave as a type I cryoglobulin in up to monoclonal IgMκ and usually also exhibit reactivity with other gly-
20% of patients, and it leads to no symptoms in most cases. 16,64,69,70 coproteins or glycolipids that share antigenic determinants with
Cryoprecipitation is dependent mainly on the concentration of MAG. 77–79 The anti–MAG-related neuropathy is typically distal and
monoclonal IgM; for this reason, plasmapheresis or plasma exchange symmetrical, affecting both motor and sensory functions; it is slowly
is commonly effective in this condition. Symptoms result from progressive with a long period of stability. 72,80 Most patients present
impaired blood flow in small vessels and include Raynaud phenom- with sensory complaints (paresthesias, aching discomfort, dysesthe-
enon; acrocyanosis; necrosis of the regions most exposed to cold, such sias, or lancinating pains); imbalance and gait ataxia, owing to lack
as the tips of the nose, ears, fingers, and toes (Fig. 87.5); malleolar proprioception; and leg muscle atrophy in advanced stage. Patients
ulcers; purpura; and cold urticaria. Renal manifestations are infre- with predominantly demyelinating sensory neuropathy in association
quent. Mixed cryoglobulins (type II) consisting of IgM-IgG complexes with monoclonal IgM to gangliosides with disialosyl moieties, such
may be associated with HCV infection. 70 as GD1b, GD3, GD2, GT1b, and GQ1b, have also been reported. 81,82
Anti-GD1b and anti-GQ1b antibodies were associated with sensory
Autoantibody Activity. Monoclonal IgM may exert its pathogenic ataxic neuropathy. These antiganglioside monoclonal IgMs present
effects through specific recognition of autologous antigens, the most core clinical features of chronic ataxic neuropathy sometimes associ-
notable being nerve constituents, immunoglobulin determinants, ated with ophthalmoplegia and/or red blood cell cold agglutinating
and red blood cell antigens. activity. The disialosyl epitope is also present on red blood cell gly-
cophorins, thereby accounting for the red cell cold agglutinin activity
IgM-Related Neuropathy. IgM-related peripheral neuropathy is of anti-Pr2 specificity. 83,84 Monoclonal IgM proteins that bind to
common in patients with WM, with estimated prevalence rates of gangliosides with a terminal trisaccharide moiety, including ganglio-
5% to 40%. 71–73 Approximately 8% of idiopathic neuropathies are side M 2 and GalNac-GD1A, are associated with chronic demyelinat-
associated with a monoclonal gammopathy, with a preponderance of ing neuropathy and severe sensory ataxia, unresponsive to
85
IgM (60%), followed by IgG (30%) and IgA (10%). 74,75 The nerve glucocorticoids. Antiganglioside IgM proteins may also cross-react
damage is mediated by diverse pathogenetic mechanisms: (1) IgM with lipopolysaccharides of Campylobacter jejuni, an infection known
antibody activity toward nerve constituents causing demyelinating to precipitate the Miller Fisher syndrome, a variant of Guillain-Barré