Page 1888 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1888
1670 Part X Transplantation
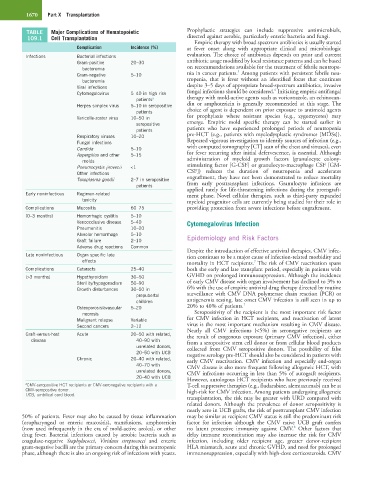
TABLE Major Complications of Hematopoietic Prophylactic strategies can include suppressive antimicrobials,
109.1 Cell Transplantation directed against aerobic, particularly enteric bacteria and fungi.
Empiric therapy with broad spectrum antibiotics is usually started
Complication Incidence (%) at fever onset along with appropriate clinical and microbiologic
Infections Bacterial infections evaluation. The choice of antibiotics depends on prior and current
Gram-positive 20–30 antibiotic usage modified by local resistance patterns and can be based
bacteremia on recommendations available for the treatment of febrile neutrope-
5
Gram-negative 5–10 nia in cancer patients. Among patients with persistent febrile neu-
bacteremia tropenia, that is fever without an identified focus that continues
Viral infections despite 3–5 days of appropriate broad-spectrum antibiotics, invasive
6
Cytomegalovirus 5–40 in high risk fungal infections should be considered. Initiating empiric antifungal
patients a therapy with mold-active agents such as voriconazole, an echinocan-
Herpes simplex virus 5–10 in seropositive din or amphotericin is generally recommended at this stage. The
patients choice of agent is dependent on prior exposure to antimold agents
Varicella-zoster virus 10–50 in for prophylaxis where resistant species (e.g., zygomycetes) may
seropositive emerge. Empiric mold specific therapy can be started earlier in
patients patients who have experienced prolonged periods of neutropenia
Respiratory viruses 10–20 pre-HCT (e.g., patients with myelodysplastic syndromes [MDSs]).
Fungal infections Repeated vigorous investigation to identify sources of infection (e.g.,
Candida 5–10 with computed tomography [CT] scan of the chest and sinuses), even
Aspergillus and other 5–15 for fever recurring after initial defervescence, is essential. Although
molds administration of myeloid growth factors (granulocyte colony-
Pneumocystis jirovecii <1 stimulating factor [G-CSF] or granulocyte-macrophage CSF [GM-
Other infections CSF]) reduces the duration of neutropenia and accelerates
Toxoplasma gondii 2–7 in seropositive engraftment, they have not been demonstrated to reduce mortality
patients from early posttransplant infections. Granulocyte infusions are
applied rarely for life-threatening infections during the preengraft-
Early noninfectious Regimen-related ment phase. Novel cellular therapies, such as third-party expanded
toxicity myeloid progenitor cells are currently being studied for their role in
Complications Mucositis 60–75 providing protection from severe infections before engraftment.
(0–3 months) Hemorrhagic cystitis 5–10
Venoocclusive disease 5–40 Cytomegalovirus Infection
Pneumonitis 10–20
Alveolar hemorrhage 5–10 Epidemiology and Risk Factors
Graft failure 2–10
Adverse drug reactions Common
Despite the introduction of effective antiviral therapies, CMV infec-
Late noninfectious Organ specific late tion continues to be a major cause of infection-related morbidity and
effects mortality in HCT recipients. The risk of CMV reactivation spans
7
Complications Cataracts 25–40 both the early and late transplant period, especially in patients with
(>3 months) Hypothyroidism 30–50 GVHD on prolonged immunosuppression. Although the incidence
Sterility/hypogonadism 50–90 of early CMV disease with organ involvement has declined to 3% to
Growth disturbances 30–50 in 6% with the use of empiric antiviral drug therapy directed by routine
prepubertal surveillance with CMV DNA polymerase chain reaction (PCR) or
children antigenemia testing, late onset CMV infection is still seen in up to
7
Osteoporosis/avascular 5–20 20% to 40% of patients.
necrosis Seropositivity of the recipient is the most important risk factor
Malignant relapse Variable for CMV infection in HCT recipients, and reactivation of latent
Second cancers 2–12 virus is the most important mechanism resulting in CMV disease.
Nearly all CMV infections (<5%) in seronegative recipients are
Graft-versus-host Acute 20–50 with related, the result of exogenous exposure (primary CMV infection), either
disease 40–90 with from a seropositive stem cell donor or from cellular blood products
unrelated donors, collected from CMV seropositive donors. The possibility of false
20–50 with UCB negative serology pre-HCT should also be considered in patients with
Chronic 20–40 with related, early CMV reactivation. CMV infection and especially end-organ
40–70 with CMV disease is also more frequent following allogeneic HCT, with
unrelated donors, CMV infections occurring in less than 5% of autograft recipients.
20–40 with UCB However, autologous HCT recipients who have previously received
a CMV-seropositive HCT recipients or CMV-seronegative recipients with a T-cell suppressive therapies (e.g., fludarabine, alemtuzumab) can be at
CMV-seropositive donor. high-risk for CMV infection. Among patients undergoing allogeneic
UCB, umbilical cord blood.
transplantation, the risk may be greater with URD compared with
related donors. Although the prevalence of donor seropositivity is
nearly zero in UCB grafts, the risk of posttransplant CMV infection
50% of patients. Fever may also be caused by tissue inflammation may be similar as recipient CMV status is still the predominant risk
(oropharyngeal or enteric mucositis), transfusions, amphotericin factor for infection although the CMV naive UCB graft confers
8
(now used infrequently in the era of mold-active azoles), or other no latent protective immunity against CMV. Other factors that
drug fever. Bacterial infections caused by aerobic bacteria such as delay immune reconstitution may also increase the risk for CMV
coagulase-negative Staphylococci, Viridans streptococci and enteric infection, including older recipient age, greater donor-recipient
gram-negative bacilli are the primary concern during this neutropenic HLA mismatch, acute and chronic GVHD, and need for prolonged
phase, although there is also an ongoing risk of infections with yeasts. immunosuppression, especially with high-dose corticosteroids. CMV