Page 1890 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 1890
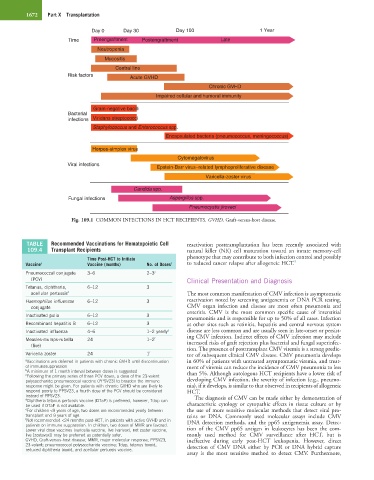
1672 Part X Transplantation
Day 0 Day 30 Day 100 1 Year
Time Preengraftment Postengraftment Late
Neutropenia
Mucositis
Central line
Risk factors Acute GVHD
Chronic GVHD
Impaired cellular and humoral immunity
Gram-negative bacilli
Bacterial
infections Viridans streptococci
Staphylococcus and Enterococcus spp.
Encapsulated bacteria (pneumococcus, meningococcus)
Herpes-simplex virus
Cytomegalovirus
Viral infections
Epstein-Barr virus–related lymphoproliferative disease
Varicella-zoster virus
Candida spp.
Fungal infections Aspergillus spp.
Pneumocystis jiroveci
Fig. 109.1 COMMON INFECTIONS IN HCT RECIPIENTS. GVHD, Graft-versus-host disease.
TABLE Recommended Vaccinations for Hematopoietic Cell reactivation posttransplantation has been recently associated with
109.4 Transplant Recipients natural killer (NK) cell maturation toward an innate memory-cell
Time Post-HCT to Initiate phenotype that may contribute to both infection control and possibly
9
Vaccine a Vaccine (months) No. of Doses b to reduced cancer relapse after allogeneic HCT.
Pneumococcal conjugate 3–6 2–3 c
(PCV) Clinical Presentation and Diagnosis
Tetanus, diphtheria, 6–12 3
acellular pertussis d The most common manifestation of CMV infection is asymptomatic
Haemophilus influenzae 6–12 3 reactivation noted by screening antigenemia or DNA PCR testing.
conjugate CMV organ infection and disease are most often pneumonia and
enteritis. CMV is the most common specific cause of interstitial
Inactivated polio 6–12 3
pneumonitis and is responsible for up to 50% of all cases. Infection
Recombinant hepatitis B 6–12 3 at other sites such as retinitis, hepatitis and central nervous system
Inactivated influenza 4–6 1–2 yearly e disease are less common and are usually seen in late-onset or persist-
ing CMV infection. Indirect effects of CMV infection may include
Measles-mumps-rubella 24 1–2 f increased risks of graft rejection plus bacterial and fungal superinfec-
(live)
tion. The presence of posttransplant CMV viremia is a strong predic-
Varicella zoster 24 1 f tor of subsequent clinical CMV disease. CMV pneumonia develops
a Vaccinations are deferred in patients with chronic GVHD until discontinuation in 60% of patients with untreated asymptomatic viremia, and treat-
of immunosuppression ment of viremia can reduce the incidence of CMV pneumonia to less
b A minimum of 1 month interval between doses is suggested
c Following the primary series of three PCV doses, a dose of the 23-valent than 5%. Although autologous HCT recipients have a lower risk of
polysaccharide pneumococcal vaccine (PPSV23) to broaden the immune developing CMV infection, the severity of infection (e.g., pneumo-
response might be given. For patients with chronic GVHD who are likely to nia), if it develops, is similar to that observed in recipients of allogeneic
respond poorly to PPSV23, a fourth dose of the PCV should be considered HCT.
instead of PPSV23. The diagnosis of CMV can be made either by demonstration of
d Diphtheria tetanus pertussis vaccine (DTaP) is preferred, however, Tdap can
be used if DTaP is not available. characteristic cytology or cytopathic effects in tissue culture or by
e For children <9 years of age, two doses are recommended yearly between the use of more sensitive molecular methods that detect viral pro-
transplant and 9 years of age. teins or DNA. Commonly used molecular assays include CMV
f Not recommended <24 months post-HCT, in patients with active GVHD and in DNA detection methods, and the pp65 antigenemia assay. Detec-
patients on immune suppression. In children, two doses of MMR are favored.
Lower viral dose vaccines (varicella vaccine, live [varivax], not zoster vaccine, tion of the CMV pp65 antigen in leukocytes has been the com-
live [zostavax]) may be preferred as potentially safer. monly used method for CMV surveillance after HCT, but is
GVHD, Graft-versus-host disease; MMR, major molecular response; PPSV23, ineffective during early post-HCT leukopenia. However, direct
23-valent; pneumococcal polysaccharide vaccine; Tdap, tetanus toxoid, detection of CMV DNA either by PCR or DNA hybrid capture
reduced diphtheria toxoid, and acellular pertussis vaccine.
assay is the most sensitive method to detect CMV. Furthermore,