Page 2206 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2206
Chapter 131 Diseases of Platelet Number 1953
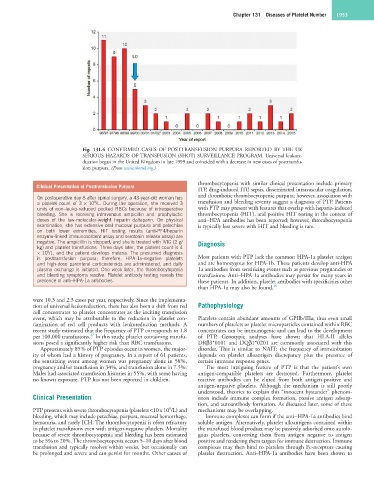
Fig. 131.4 CONFIRMED CASES OF POSTTRANSFUSION PURPURA REPORTED BY THE UK
SERIOUS HAZARDS OF TRANSFUSION (SHOT) SURVEILLANCE PROGRAM. Universal leukore-
duction began in the United Kingdom in late 1999 and coincided with a decrease in new cases of posttransfu-
sion purpura. (From www.shotuk.org.)
thrombocytopenia with similar clinical presentation include primary
Clinical Presentation of Posttransfusion Purpura
ITP, drug-induced ITP, sepsis, disseminated intravascular coagulation,
On postoperative day 8 after spinal surgery, a 43-year-old woman has and thrombotic thrombocytopenic purpura; however, association with
a platelet count of 3 × 10 /L. During the operation, she received 3 transfusion and bleeding severity suggest a diagnosis of PTP. Patients
9
units of non–leuko-reduced packed RBCs because of intraoperative with PTP may present with features that overlap with heparin-induced
bleeding. She is receiving intravenous ampicillin and prophylactic thrombocytopenia (HIT), and positive HIT testing in the context of
doses of the low-molecular-weight heparin dalteparin. On physical anti–HPA antibodies has been reported; however, thrombocytopenia
examination, she has extensive oral mucosal purpura and petechiae is typically less severe with HIT and bleeding is rare.
on both lower extremities. HIT testing results (anti-PF4/heparin
enzyme-linked immunosorbent assay and serotonin release assay) are
negative. The ampicillin is stopped, and she is treated with IVIG (2 g/ Diagnosis
kg) and platelet transfusions. Three days later, the platelet count is 4
9
× 10 /L, and the patient develops melena. The presumed diagnosis
is posttransfusion purpura; therefore, HPA-1a–negative platelets Most patients with PTP lack the common HPA-1a platelet antigen
and high-dose parenteral corticosteroids are administered, and daily and are homozygous for HPA-1b. These patients develop anti-HPA
plasma exchange is initiated. One week later, the thrombocytopenia 1a antibodies from sensitizing events such as previous pregnancies or
and bleeding symptoms resolve. Platelet antibody testing reveals the transfusions. Anti–HPA 1a antibodies may persist for many years in
presence of anti–HPA-1a antibodies. these patients. In addition, platelet antibodies with specificities other
than HPA-1a may also be found. 28
were 10.3 and 2.3 cases per year, respectively. Since the implementa-
tion of universal leukoreduction, there has also been a shift from red Pathophysiology
cell concentrates to platelet concentrates as the inciting transfusion
event, which may be attributable to the reduction in platelet con- Platelets contain abundant amounts of GPIIb/IIIa; thus even small
tamination of red cell products with leukoreduction methods. A numbers of platelets or platelet microparticles contained within RBC
recent study estimated that the frequency of PTP corresponds to 1.8 concentrates can be immunogenic and can lead to the development
27
per 100,000 transfusions. In this study, platelet containing transfu- of PTP. Genotypic analyses have shown that HLA-II alleles
sions posed a significantly higher risk than RBC transfusions. DRβ3*0101 and DQβ1*0201 are commonly associated with this
Approximately 85% of PTP episodes occur in women, the major- disorder. This is similar to NAIT: the frequency of immunization
ity of whom had a history of pregnancy. In a report of 61 patients, depends on platelet alloantigen discrepancy plus the presence of
the sensitizing event among women was pregnancy alone in 58%, certain immune response genes.
pregnancy and/or transfusion in 34%, and transfusion alone in 7.5%. The most intriguing feature of PTP is that the patient’s own
Males had associated transfusion histories in 55%, with some having antigen-compatible platelets are destroyed. Furthermore, platelet
no known exposure. PTP has not been reported in children. reactive antibodies can be eluted from both antigen-positive and
antigen-negative platelets. Although the mechanism is still poorly
understood, theories to explain this “innocent bystander” phenom-
Clinical Presentation enon include immune complex formation, passive antigen adsorp-
tion, and autoantibody formation. As discussed later, some of these
9
PTP presents with severe thrombocytopenia (platelets <10 x 10 /L) and mechanisms may be overlapping.
bleeding, which may include petechiae, purpura, mucosal hemorrhage, Immune complexes can form if the anti–HPA-1a antibodies bind
hematuria, and rarely ICH. The thrombocytopenia is often refractory soluble antigen. Alternatively, platelet alloantigens contained within
to platelet transfusions even with antigen-negative platelets. Mortality the transfused blood product may be passively adsorbed onto autolo-
because of severe thrombocytopenia and bleeding has been estimated gous platelets, converting them from antigen negative to antigen
to be 5% to 20%. The thrombocytopenia occurs 5–10 days after blood positive and rendering them targets for immune destruction. Immune
transfusion and typically resolves within weeks, but occasionally can complexes may then bind to platelets through Fc-receptors causing
be prolonged and severe and can persist for months. Other causes of platelet destruction. Anti–HPA-1a antibodies have been shown to