Page 2348 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2348
2090 Part XII Hemostasis and Thrombosis
Small angle
Crystal structure Electron microscopy
X-ray scattering
Bouma et al.; Schwarzenbacher et al. Hammel et al. Agar et al.
Epitope R39-R43 available
for binding antibodies
Domain V with positive charge Phospholipid Negatively charged
carbohydrate chain
Domain I and II with Anionic phospholipid
positive-charged epitope
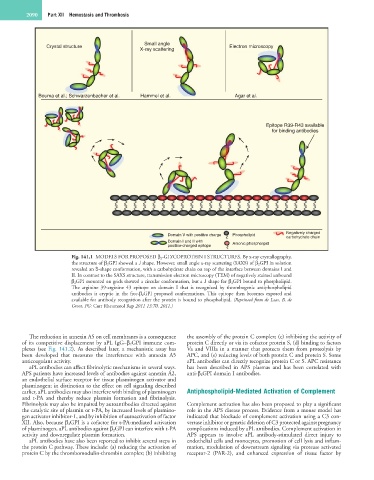
Fig. 141.1 MODELS FOR PROPOSED β 2-GLYCOPROTEIN I STRUCTURES. By x-ray crystallography,
the structure of β 2GPI showed a J shape. However, small angle x-ray scattering (SAXS) of β 2GPI in solution
revealed an S-shape conformation, with a carbohydrate chain on top of the interface between domains I and
II. In contrast to the SAXS structure, transmission electron microscopy (TEM) of negatively stained unbound
β 2GPI mounted on grids showed a circular conformation, but a J shape for β 2GPI bound to phospholipid.
The arginine 39-arginine 43 epitope on domain I that is recognized by thrombogenic antiphospholipid
antibodies is cryptic in the free-β 2GPI proposed conformations. This epitope then becomes exposed and
available for antibody recognition after the protein is bound to phospholipid. (Reprinted from de Laat, B, de
Groot, PG: Curr Rheumatol Rep 2011 13:70, 2011.)
The reduction in annexin A5 on cell membranes is a consequence the assembly of the protein C complex; (c) inhibiting the activity of
of its competitive displacement by aPL IgG–β 2 GPI immune com- protein C directly or via its cofactor protein S, (d) binding to factors
plexes (see Fig. 141.2). As described later, a mechanistic assay has Va and VIIIa in a manner that protects them from proteolysis by
been developed that measures the interference with annexin A5 APC, and (e) reducing levels of both protein C and protein S. Some
anticoagulant activity. aPL antibodies can directly recognize protein C or S. APC resistance
aPL antibodies can affect fibrinolytic mechanisms in several ways. has been described in APS plasmas and has been correlated with
APS patients have increased levels of antibodies against annexin A2, anti-β 2 GPI domain I antibodies.
an endothelial surface receptor for tissue plasminogen activator and
plasminogen; in distinction to the effect on cell signaling described
earlier, aPL antibodies may also interfere with binding of plasminogen Antiphospholipid-Mediated Activation of Complement
and t-PA and thereby reduce plasmin formation and fibrinolysis.
Fibrinolysis may also be impaired by autoantibodies directed against Complement activation has also been proposed to play a significant
the catalytic site of plasmin or t-PA, by increased levels of plasmino- role in the APS disease process. Evidence from a mouse model has
gen activator inhibitor-1, and by inhibition of autoactivation of factor indicated that blockade of complement activation using a C3 con-
XII. Also, because β 2GPI is a cofactor for t-PA-mediated activation vertase inhibitor or genetic deletion of C3 protected against pregnancy
of plasminogen, aPL antibodies against β 2GPI can interfere with t-PA complications induced by aPL antibodies. Complement activation in
activity and downregulate plasmin formation. APS appears to involve aPL antibody-stimulated direct injury to
aPL antibodies have also been reported to inhibit several steps in endothelial cells and monocytes, promotion of cell lysis and inflam-
the protein C pathway. These include: (a) reducing the activation of mation, modulation of downstream signaling via protease activated
protein C by the thrombomodulin-thrombin complex; (b) inhibiting receptor-2 (PAR-2), and enhanced expression of tissue factor by