Page 2447 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2447
Chapter 149 Antithrombotic Drugs 2185
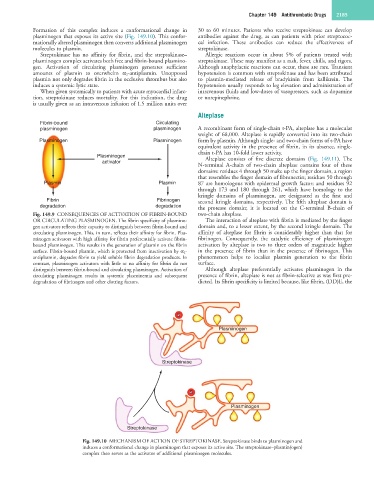
Formation of this complex induces a conformational change in 30 to 60 minutes. Patients who receive streptokinase can develop
plasminogen that exposes its active site (Fig. 149.10). This confor- antibodies against the drug, as can patients with prior streptococ-
mationally altered plasminogen then converts additional plasminogen cal infection. These antibodies can reduce the effectiveness of
molecules to plasmin. streptokinase.
Streptokinase has no affinity for fibrin, and the streptokinase– Allergic reactions occur in about 5% of patients treated with
plasminogen complex activates both free and fibrin-bound plasmino- streptokinase. These may manifest as a rash, fever, chills, and rigors.
gen. Activation of circulating plasminogen generates sufficient Although anaphylactic reactions can occur, these are rare. Transient
amounts of plasmin to overwhelm α 2-antiplasmin. Unopposed hypotension is common with streptokinase and has been attributed
plasmin not only degrades fibrin in the occlusive thrombus but also to plasmin-mediated release of bradykinin from kallikrein. The
induces a systemic lytic state. hypotension usually responds to leg elevation and administration of
When given systemically to patients with acute myocardial infarc- intravenous fluids and low-doses of vasopressors, such as dopamine
tion, streptokinase reduces mortality. For this indication, the drug or norepinephrine.
is usually given as an intravenous infusion of 1.5 million units over
Alteplase
Fibrin-bound Circulating
plasminogen plasminogen A recombinant form of single-chain t-PA, alteplase has a molecular
weight of 68,000. Alteplase is rapidly converted into its two-chain
Plasminogen Plasminogen form by plasmin. Although single- and two-chain forms of t-PA have
equivalent activity in the presence of fibrin, in its absence, single-
chain t-PA has 10-fold lower activity.
Plasminogen Alteplase consists of five discrete domains (Fig. 149.11). The
activator
N-terminal A-chain of two-chain alteplase contains four of these
domains: residues 4 through 50 make up the finger domain, a region
that resembles the finger domain of fibronectin; residues 50 through
Plasmin Plasmin 87 are homologous with epidermal growth factor; and residues 92
through 173 and 180 through 261, which have homology to the
kringle domains of plasminogen, are designated as the first and
Fibrin Fibrinogen second kringle domains, respectively. The fifth alteplase domain is
degradation degradation the protease domain; it is located on the C-terminal B-chain of
Fig. 149.9 CONSEQUENCES OF ACTIVATION OF FIBRIN-BOUND two-chain alteplase.
OR CIRCULATING PLASMINOGEN. The fibrin specificity of plasmino- The interaction of alteplase with fibrin is mediated by the finger
gen activators reflects their capacity to distinguish between fibrin-bound and domain and, to a lesser extent, by the second kringle domain. The
circulating plasminogen. This, in turn, reflects their affinity for fibrin. Plas- affinity of alteplase for fibrin is considerably higher than that for
minogen activators with high affinity for fibrin preferentially activate fibrin- fibrinogen. Consequently, the catalytic efficiency of plasminogen
bound plasminogen. This results in the generation of plasmin on the fibrin activation by alteplase is two to three orders of magnitude higher
surface. Fibrin-bound plasmin, which is protected from inactivation by α 2 - in the presence of fibrin than in the presence of fibrinogen. This
antiplasmin, degrades fibrin to yield soluble fibrin degradation products. In phenomenon helps to localize plasmin generation to the fibrin
contrast, plasminogen activators with little or no affinity for fibrin do not surface.
distinguish between fibrin-bound and circulating plasminogen. Activation of Although alteplase preferentially activates plasminogen in the
circulating plasminogen results in systemic plasminemia and subsequent presence of fibrin, alteplase is not as fibrin-selective as was first pre-
degradation of fibrinogen and other clotting factors. dicted. Its fibrin specificity is limited because, like fibrin, (DD)E, the
S
Plasminogen
Streptokinase
S
Plasminogen
Streptokinase
Fig. 149.10 MECHANISM OF ACTION OF STREPTOKINASE. Streptokinase binds to plasminogen and
induces a conformational change in plasminogen that exposes its active site. The streptokinase–plasmin(ogen)
complex then serves as the activator of additional plasminogen molecules.