Page 2557 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2557
2280 Part XIII Consultative Hematology
Role of the Spleen
A E Some degree of splenomegaly is a normal feature of malarial infec-
tion, and the prevalence of splenomegaly in regions of malarial
transmission is used as a major indicator of the level of malarial
endemicity. The importance of the spleen in host defense against
malaria has been demonstrated in experimental systems, and indi-
LIVER viduals whose spleens have been surgically removed are thought to
be more susceptible to severe infection. Indeed, the phenomenon of
B D parasitic sequestration is thought to have evolved primarily as an
immune evasion strategy so the mature parasite can avoid passing
through the spleen. 27
Active erythrophagocytosis is a conspicuous feature within the
bone marrow during P. vivax and P. falciparum malaria, 28,29 and it
Asexual is highly probable that this also occurs within the spleen. Cytokines
growth may be responsible for activating macrophages during malarial
infection. Children with acute P. falciparum malaria have high cir-
C culating levels of interferon-γ (IFN-γ) and tumor necrosis factor-α
30
Adhesion to (TNF-α), a synergistic combination of cytokines that activates
endothelial and macrophages.
other host cells Researchers in several studies have attempted to define the patho-
physiologic changes in the spleen during acute malaria. In animal
models, malaria is accompanied by increased intravascular clearance
31
of infected or rigid, heat-treated cells by the spleen, as well as altera-
KEY: 32
tions in the splenic microcirculation. In studies of human malaria,
RBC Merozoites Ring Trophozoites/ it has been found that increased splenic clearance of heated RBCs
schizonts occurs during acute attacks. More recent, histologic studies and ex
33
vivo models of splenic function suggest that the spleen removes not
only mature infected RBCs but also uninfected cells marked for
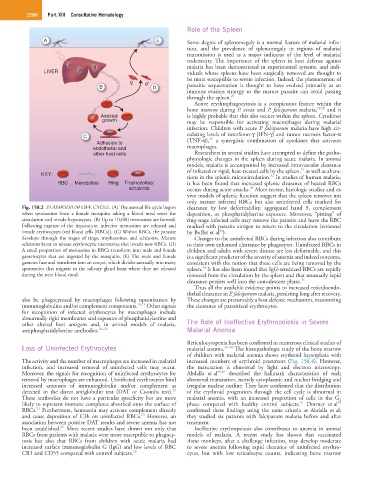
Fig. 158.2 PLASMODIUM LIFE CYCLE. (A) The asexual life cycle begins clearance by low deformability, aggregated band 3, complement
when sporozoites from a female mosquito taking a blood meal enter the deposition, or phosphatidylserine exposure. Moreover, “pitting” of
circulation and invade hepatocytes. (B) Up to 10,000 merozoites are formed. ring-stage infected cells may remove the parasite and leave the RBC
Following rupture of the hepatocyte, infective merozoites are released and marked with parasite antigen to return to the circulation (reviewed
invade erythrocytes (red blood cells [RBCs]). (C) Within RBCs, the parasite by Buffet et al ).
34
develops through the stages of rings, trophozoites, and schizonts. Mature Changes to the uninfected RBCs during infection also contribute
schizonts burst to release erythrocytic merozoites that invade new RBCs. (D) to their own enhanced clearance by phagocytes. Uninfected RBCs in
A small proportion of merozoites in RBCs transform into male and female children and adults with severe disease are less deformable, and this
gametocytes that are ingested by the mosquito. (E) The male and female is a significant predictor of the severity of anemia and indeed outcome,
gametes fuse and transform into an oocyst, which divides asexually into many consistent with the notion that these cells are being removed by the
sporozoites that migrate to the salivary gland from where they are released spleen. It has also been found that IgG-sensitized RBCs are rapidly
20
during the next blood meal. removed from the circulation by the spleen and that unusually rapid
clearance persists well into the convalescent phase. 35
Thus all the available evidence points to increased reticuloendo-
thelial clearance in P. falciparum malaria, persisting long after recovery.
also be phagocytosed by macrophages following opsonization by These changes are presumably a host defense mechanism, maximizing
immunoglobulins and/or complement components. 18,19 Other signals the clearance of parasitized erythrocytes.
for recognition of infected erythrocytes by macrophages include
abnormally rigid membranes and exposure of phosphatidylserine and
other altered host antigens and, in animal models of malaria, The Role of Ineffective Erythropoiesis in Severe
antiphosphatidylserine antibodies. 20–23 Malarial Anemia
Reticulocytopenia has been confirmed in numerous clinical studies of
Loss of Uninfected Erythrocytes malarial anemia. 36–38 The histopathologic study of the bone marrow
of children with malarial anemia shows erythroid hyperplasia with
The activity and the number of macrophages are increased in malarial increased numbers of erythroid precursors (Fig. 158.4). However,
infection, and increased removal of uninfected cells may occur. the maturation is abnormal by light and electron microscopy.
Moreover, the signals for recognition of uninfected erythrocytes for Abdalla et al 39,40 described the hallmark characteristics of such
removal by macrophages are enhanced. Uninfected erythrocytes bind abnormal maturation, namely cytoplasmic and nuclear bridging and
increased amounts of immunoglobulin and/or complement as irregular nuclear outline. They later confirmed that the distribution
23
detected in the direct antiglobulin test (DAT or Coombs test). of the erythroid progenitors through the cell cycle is abnormal in
These antibodies do not have a particular specificity but are more malarial anemia, with an increased proportion of cells in the G 2
38
41
likely to represent immune complexes absorbed onto the surface of phase compared with healthy control subjects. Dormer et al
23
RBCs. Furthermore, hemozoin may activate complement directly confirmed these findings using the same criteria as Abdalla et al;
24
and cause deposition of C3b on uninfected RBCs. However, an they studied six patients with falciparum malaria before and after
association between positive DAT results and severe anemia has not treatment.
25
been established. More recent studies have shown not only that Ineffective erythropoiesis also contributes to anemia in animal
RBCs from patients with malaria were more susceptible to phagocy- models of malaria. A recent study has shown that vaccinated
tosis but also that RBCs from children with acute malaria had Aotus monkeys, after a challenge infection, may develop moderate
increased surface immunoglobulin G (IgG) and low levels of RBC to severe anemia following rapid clearance of uninfected erythro-
CR1 and CD55 compared with control subjects. 26 cytes, but with low reticulocyte counts, indicating bone marrow