Page 2558 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2558
Chapter 158 Hematologic Aspects of Parasitic Diseases 2281
RSP2 Bone marrow sinusoid Spleen
Clearance of non-iRBC
MØ
Clearance of iRBC
RSP2 immune complexes
Opsonization by specific Ab
MØ
Clearance of debris
iRBC and debris
Activation of MØ
Pigment and GPI
containing monocyte
Direct effect of Hz
parasite ++
– Indirect effect by ↑↑ TNF-α:IL-10
cytokines and ↑ MIF
+ – – other mediators ↓ IL-12
+
+
Anemia ↑↑ Epo
malaria infection
Erythropoiesis
KEY: Reticulocyte RBC iRBC iRBC
Schizont stage
Ring stage
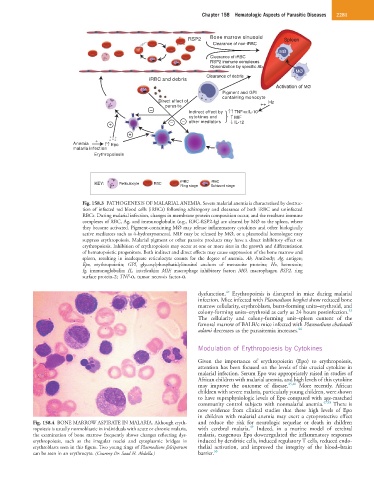
Fig. 158.3 PATHOGENESIS OF MALARIAL ANEMIA. Severe malarial anemia is characterized by destruc-
tion of infected red blood cells (iRBCs) following schizogony and clearance of both iRBC and uninfected
RBCs. During malarial infection, changes in membrane protein composition occur, and the resultant immune
complexes of RBC, Ag, and immunoglobulin (e.g., RBC-RSP2-Ig) are cleared by MØ to the spleen, where
they become activated. Pigment-containing MØ may release inflammatory cytokines and other biologically
active mediators such as 4-hydroxynonenal. MIF may be released by MØ, or a plasmodial homologue may
suppress erythropoiesis. Malarial pigment or other parasite products may have a direct inhibitory effect on
erythropoiesis. Inhibition of erythropoiesis may occur at one or more sites in the growth and differentiation
of hematopoietic progenitors. Both indirect and direct effects may cause suppression of the bone marrow and
spleen, resulting in inadequate reticulocyte counts for the degree of anemia. Ab, Antibody; Ag, antigen;
Epo, erythropoietin; GPI, glycosylphosphatidylinositol anchors of merozoite proteins; Hz, hemozoin;
Ig, immunoglobulin; IL, interleukin; MIF, macrophage inhibitory factor; MØ, macrophages; RSP2, ring
surface protein-2; TNF-α, tumor necrosis factor-α.
42
dysfunction. Erythropoiesis is disrupted in mice during malarial
infection. Mice infected with Plasmodium berghei show reduced bone
marrow cellularity, erythroblasts, burst-forming units–erythroid, and
43
colony-forming units–erythroid as early as 24 hours postinfection.
The cellularity and colony-forming unit–spleen content of the
femoral marrow of BALB/c mice infected with Plasmodium chabaudi
adami decreases as the parasitemia increases. 44
Modulation of Erythropoiesis by Cytokines
Given the importance of erythropoietin (Epo) to erythropoiesis,
attention has been focused on the levels of this crucial cytokine in
malarial infection. Serum Epo was appropriately raised in studies of
African children with malarial anemia, and high levels of this cytokine
may improve the outcome of disease. 45,46 More recently, African
children with severe malaria, particularly young children, were shown
to have supraphysiologic levels of Epo compared with age-matched
community control subjects with nonmalarial anemia. 47,48 There is
now evidence from clinical studies that these high levels of Epo
in children with malarial anemia may exert a cytoprotective effect
Fig. 158.4 BONE MARROW ASPIRATE IN MALARIA. Although eryth- and reduce the risk for neurologic sequelae or death in children
49
ropoiesis is usually normoblastic in individuals with acute or chronic malaria, with cerebral malaria. Indeed, in a murine model of cerebral
the examination of bone marrow frequently shows changes reflecting dys- malaria, exogenous Epo downregulated the inflammatory responses
erythropoiesis, such as the irregular nuclei and cytoplasmic bridges in induced by dendritic cells, induced regulatory T cells, reduced endo-
erythroblasts seen in this figure. Two young rings of Plasmodium falciparum thelial activation, and improved the integrity of the blood–brain
can be seen in an erythrocyte. (Courtesy Dr. Saad H. Abdalla.) barrier. 50