Page 2562 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 2562
Chapter 158 Hematologic Aspects of Parasitic Diseases 2285
In endemic areas, laboratory staff are skilled at the examination must maintain the skills needed for reliable examination of thick
of thick films and routinely are able to detect 1 parasite in 100 films.
high-power fields of a thick film, which corresponds to a sensitivity A number of methods based on the fluorescent staining of parasite
of approximately 5 to 50 parasites/µL. 107,110 Thin films are used for deoxyribonucleic acid (DNA) and/or ribonucleic acid (RNA) and the
determining the species of the parasites, and the circulating asexual concentration of parasites have been devised. 112–114 However attractive
forms of the four main malaria species can be readily identified, these methods may appear, their sensitivity is limited by background
whereas the sexual forms (gametocytes) of the species require some staining of cellular debris, and the limit of their sensitivity is approxi-
skill and regular practice (Figs. 158.5 and 158.6). mately 100 parasites/µL. The time taken in preparing samples and
Nevertheless, diagnosis of malaria by microscopy in nonendemic the specialized equipment and skills needed to use these methods
countries has proven problematic. Routine laboratories may only limit their effectiveness in routine practice.
achieve sensitivities of the order of 500 parasites/µL using thick Detection of circulating malarial antigens is another potentially
films. 110,111 Quality assurance schemes show that performance of attractive, but ultimately limited, alternative to the laborious method
routine hematology laboratories in the recognition of and species of screening blood films. The widely available tests detect Plasmodium
determination of malaria parasites is poor. 109,111 histidine-rich protein 2 (BinaxNOW Malaria test) and Plasmodium-
There has therefore been a strong drive to use nonmicroscopic specific lactate dehydrogenase (OptiMal-IT test) by immunochro-
115
methods for malaria diagnosis. It is now apparent that these methods matography. The formulation of the tests using dipstick antigens
are not sufficiently sensitive for clinical diagnosis, although they may allows rapid testing to be performed by laboratory staff. However, the
have a role in detecting parasites of more than 500 parasites/µL when sensitivity is variable and may range from 100 to 1000 parasites/µL,
experienced staff are not available and/or as part of an out-of-hours and this is comparable to the sensitivity achieved by inexperienced
service. However, it must be emphasized that these tests are no microscopists and may approach that achieved by experienced
substitute for careful microscopy. Operationally, this means that microscopists. The current recommendations for malaria diagnosis in
hematology laboratories need to make the diagnosis of malaria and the United Kingdom emphasize that the optimum diagnostic
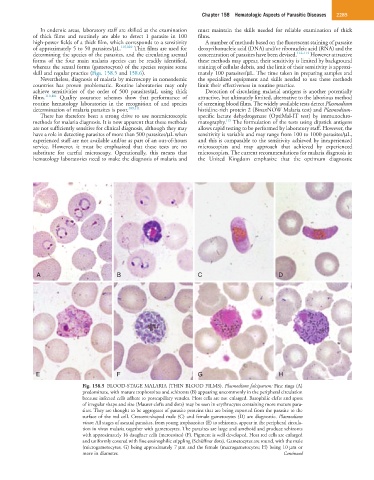
A B C D
E F G H
Fig. 158.5 BLOOD-STAGE MALARIA (THIN BLOOD FILMS). Plasmodium falciparum: Fine rings (A)
predominate, with mature trophozoites and schizonts (B) appearing uncommonly in the peripheral circulation
because infected cells adhere to postcapillary venules. Host cells are not enlarged. Basophilic clefts and spots
of irregular shape and size (Maurer clefts and dots) may be seen in erythrocytes containing more mature para-
sites. They are thought to be aggregates of parasite proteins that are being exported from the parasite to the
surface of the red cell. Crescent-shaped male (C) and female gametocytes (D) are diagnostic. Plasmodium
vivax: All stages of asexual parasites, from young trophozoites (E) to schizonts, appear in the peripheral circula-
tion in vivax malaria together with gametocytes. The parasites are large and ameboid and produce schizonts
with approximately 16 daughter cells (merozoites) (F). Pigment is well developed. Host red cells are enlarged
and uniformly covered with fine eosinophilic stippling (Schüffner dots). Gametocytes are round, with the male
(microgametocytes; G) being approximately 7 µm and the female (macrogametocytes; H) being 10 µm or
more in diameter. Continued