Page 300 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 300
248 Part III Immunologic Basis of Hematology
These cells originate in the lamina propria and migrate to the mesen-
teric lymph nodes, where they drive the differentiation of gut-homing
+
FoxP3 regulatory T cells (Tregs) by producing retinoic acid (RA)
from dietary vitamin A.
+
+
In addition, the BDCA3 (DC1 CD141 ) DC subset has been
+
found to be the equivalent of murine CD8α DCs, and are involved
+
at cross-presenting antigens to CD8 T cells. They express the
chemokine receptor XCR1 and the DC NK lectin group receptor 1
C-type lectin, a sensor for necrotic cells, and specifically β-actin, and
they mediate the phagocytosis of dead cells. They also express basic
leucine zipper transcriptional factor ATF-like-3 (BATF3) and INF
regulatory factor-8 (IRF8), which may be essential for their develop-
+
ment. BDCA3 DCs express high levels of TLR3 and TLR8, and,
upon stimulation by TLR3 agonists (e.g., polyinosinic-polycytidylic
acid [poly(I:C)]), they secrete high amounts of IL-12 and IFN-β,
both Th1-skewing cytokines. These combined characteristics make
them attractive targets for DC-based vaccines in cancer and chronic
immune diseases.
Although Langerhans cells and microglia seem to be capable of
self-renewal in ectodermal tissues, epidermis, and brain, other DCs
arise from blood-borne precursors from BM as described earlier.
Recent studies of human immunodeficiencies have highlighted the
transcription factors directing the development of DCs and have
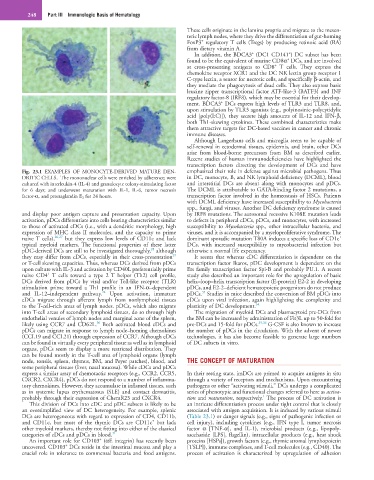
Fig. 23.1 EXAMPLES OF MONOCYTE-DERIVED MATURE DEN- emphasized their role in defense against microbial pathogens. Thus
DRITIC CELLS. The mononuclear cells were enriched by adherence; were in DC, monocyte, B, and NK lymphoid deficiency (DCML), blood
cultured with interleukin-4 (IL-4) and granulocyte colony-stimulating factor and interstitial DCs are absent along with monocytes and pDCs.
for 6 days; and underwent maturation with IL-1, IL-6, tumor necrosis The DCML is attributable to GATA-binding factor 2 mutations, a
factor-α, and prostaglandin E 2 for 24 hours. transcription factor involved in the homeostasis of HSCs. Patients
with DCML deficiency have increased susceptibility to Mycobacteria
spp., fungi, and viruses. Another DC deficiency syndrome is caused
and display poor antigen capture and presentation capacity. Upon by IRF8 mutations. The autosomal recessive K108E mutation leads
activation, pDCs differentiate into cells bearing characteristics similar to defects in peripheral cDCs, pDCs, and monocytes, with increased
to those of activated cDCs (i.e., with a dendritic morphology, high susceptibility to Mycobacteria spp., other intracellular bacteria, and
expression of MHC class II molecules, and the capacity to prime viruses, and it is accompanied by a myeloproliferative syndrome. The
+
naive T cells), 26,27 but they express low levels of CD11c and lack dominant sporadic mutation T80A induces a specific loss of CD1c
typical myeloid markers. The functional properties of these latter DCs, with increased susceptibility to mycobacterial infection but
28
pDC-derived DCs are still to be investigated thoroughly, although otherwise a normal life expectancy.
29
they may differ from cDCs, especially in their cross-presentation It seems that whereas cDC differentiation is dependent on the
or T-cell skewing capacities. Thus, whereas DCs derived from pDCs transcription factor Ikaros, pDC development is dependent on the
upon culture with IL-3 and activation by CD40L preferentially prime Ets family transcription factor Spi-B and probably PU.1. A recent
+
naive CD4 T cells toward a type 2 T helper (Th2) cell profile, study also described an important role for the upregulation of basic
DCs derived from pDCs by viral and/or Toll-like receptor (TLR) helix–loop–helix transcription factor (E-protein) E2-2 in developing
stimulation prime toward a Th1 profile in an IFN-α–dependent pDCs, and E2-2–deficient hematopoietic progenitors do not produce
33
24
and IL-12–independent pathway. Upon activation, immature pDCs. Studies in mice described the conversion of BM pDCs into
cDCs migrate through afferent lymph from nonlymphoid tissues cDCs upon viral infection, again highlighting the complexity and
to the T-cell–rich areas of lymph nodes. pDCs, which also migrate plasticity of DC development. 34
into T-cell areas of secondary lymphoid tissues, do so through high The migration of myeloid DCs and plasmacytoid pre-DCs from
endothelial venules of lymph nodes and marginal zone of the spleen, the BM can be increased by administration of Flt3L up to 50-fold for
30
likely using CCR7 and CD62L. Both activated blood cDCs and pre-DCs and 15-fold for pDCs. 35,36 G-CSF is also known to increase
pDCs can migrate in response to lymph node–homing chemokines the number of pDCs in the circulation. With the advent of newer
(CCL19 and CCL21) through expression of CCR7. Although cDCs technologies, it has also become feasible to generate large numbers
can be found in virtually every peripheral tissue as well as in lymphoid of DC subsets in vitro.
organs, pDCs seem to display a more restricted distribution. They
can be found mostly in the T-cell area of lymphoid organs (lymph
node, tonsils, spleen, thymus, BM, and Peyer patches), blood, and THE CONCEPT OF MATURATION
some peripheral tissues (liver, nasal mucosa). While cDCs and pDCs
express a similar array of chemotactic receptors (e.g., CCR2, CCR5, In their resting state, imDCs are primed to acquire antigens in situ
CXCR2, CXCR4), pDCs do not respond to a number of inflamma- through a variety of receptors and mechanisms. Upon encountering
tory chemokines. However, they accumulate in inflamed tissues, such pathogens or other “activating stimuli,” DCs undergo a complicated
as in systemic lupus erythematosus (SLE) and contact dermatitis, series of phenotypic and functional changes referred to here as activa-
1
probably through their expression of ChemR23 and CXCR4. tion and maturation, respectively. The process of DC activation is
This division of DCs into cDC and pDC subsets is likely to be an intricate differentiation process under tight control that is closely
an oversimplified view of DC heterogeneity. For example, splenic associated with antigen acquisition. It is induced by various stimuli
DCs are heterogeneous with regard to expression of CD4, CD11b, (Table 23.1) or danger signals (e.g., signs of pathogenic infection or
+
and CD11c, but most of the thymic DCs are CD11c but lack cell injury), including cytokines (e.g., IFN type I, tumor necrosis
other myeloid markers, thereby not fitting into either of the classical factor α [TNF-α], and IL-1), microbial products (e.g., lipopoly-
categories of cDCs and pDCs in blood. 31 saccharide [LPS], flagellin), intracellular products (e.g., heat shock
+
An important role for CD103 (αE integrin) has recently been proteins [HSPs]), growth factors (e.g., thymic stromal lymphopoietin
+
uncovered. CD103 DCs reside in the intestinal mucosa and play a [TSLP]), immune complexes, and T-cell molecules (e.g., CD40). The
crucial role in tolerance to commensal bacteria and food antigens. process of activation is characterized by upregulation of adhesion