Page 303 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 303
Chapter 23 Dendritic Cell Biology 251
as the generation of tolerogenic signals; and MMR, involved in the cells such as CD4 and CD8 T cells. Depending on the antigens the
processing of microbial organisms. DCs encounter, the presentation pathway will differ, as described in
Other receptors expressed by DCs include FcR, which is involved detail later.
in cross-presentation of immune complexes and antibody-opsonized
dead cells; integrins such as α vβ 5, scavenger receptor CD36, and
Mer family tyrosine kinases for phagocytosis of apoptotic cells and Major Histocompatibility Complex Class I Antigen
lipoxygenase-1 or CD91 for uptake of HSPs; complement receptors Presentation (Endogenous Route)
that play a role in uptake of opsonized microbes and apoptotic cells;
+
receptors for viruses (e.g., CD4, CCR5, and CXCR4 for HIV and The process of antigen processing and presentation to CD8 T cells
CD46 for measles virus); and the CD1 family of receptors that begins with degradation of proteins synthesized within the cytoplasm,
activate CD4, CD8, γδT cells, and NK T cells through binding and either as mature proteins or as neosynthesized defective proteins
processing of antigens such as sphingolipids, sulfatides, glycosphin- (defective ribosomal products), into oligopeptides by the ubiquitin–
golipids, glycosylphosphatidylinositol (GPI)-anchored mucin-like proteasome pathway. Misfolded proteins are also a source of antigenic
glycoproteins (GPI mucins), glycoinositolphospholipids, and their peptides after retrotranslocation from the ER to the cytosol through
phosphatidylinositol moieties. Altogether, these various receptors the ER-associated degradation pathway. Subsequently, aminopep-
provide substantial avenues for DCs to efficiently capture multitudes tidases cleave N-terminal precursors into peptides of appropriate
of antigens in their environment. length for presentation on MHC class I molecules. Antigen process-
Antigen capture is tightly coupled to DC activation and antigen ing via this route is regulated through activation of the catalytically
presentation, and triggering of TLR or exposure to inflammatory active subunits of the proteasome, the PA28 proteasome activator,
53
cytokines first induces a transient increase in the macropinocytic and leucine aminopeptidase, which are upregulated by IFN. mDCs
uptake followed by a nearly complete downregulation of the uptake in particular express immunoproteosomes containing the active site
process. Furthermore, it has been suggested that TLR engagement subunits latent membrane protein 2 (LMP2), LMP7, and MECL-1,
54
also enhances microbe-loaded phagosome maturation, potentially which can enhance antigen processing. After transport into the
discriminating between nonimmunogenic antigens (apoptotic cells) ER through the transporter associated with antigen processing (Fig.
and microbial antigens at the antigen-processing level. 52 23.2), long peptides are further trimmed by ER aminopeptidase-1 to
8-mer or 9-mer peptides for loading onto MHC class I molecules.
DCs also have the capacity to acquire antigens exogenously and
ANTIGEN PROCESSING process them for presentation on MHC class I molecules. This phe-
nomenon, referred to as cross-presentation, allows the immune system
DCs are highly efficient in processing and presenting antigens via to recognize antigens that are not otherwise presented or that may
their MHC or CD1 molecules and presenting those to other immune not access DCs directly (e.g., tumor cells, viruses). DCs can acquire
Phagosome
3
Phagocytosis
Proteasome
1 1 3
Processed
peptides 3a? 3
TAP 1 Endogenous/
foreign proteins 2
?
Peptide- 1 Phagosome+
MHC I ER components
Endoplasmic reticulum (ER)
1 1 2
Golgi
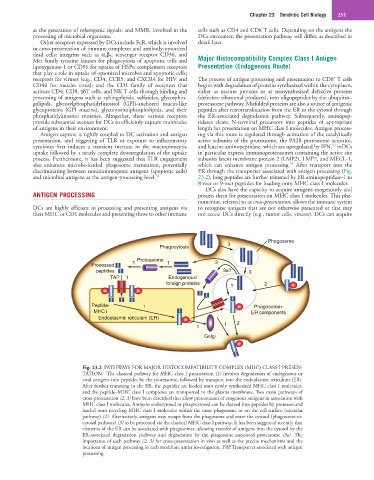
Fig. 23.2 PATHWAYS FOR MAJOR HISTOCOMPATIBILITY COMPLEX (MHC) CLASS I PRESEN-
TATION. The classical pathway for MHC class I presentation (1) involves degradation of endogenous or
viral antigens into peptides by the proteasome, followed by transport into the endoplasmic reticulum (ER).
After further trimming in the ER, the peptides are loaded onto newly synthesized MHC class I molecules,
and the peptide–MHC class I complexes are transported to the plasma membrane. Two main pathways of
cross-presentation (2, 3) have been described that allow presentation of exogenous antigens in association with
MHC class I molecules. Antigens endocytosed or phagocytosed can be cleaved into peptides by proteases and
loaded onto recycling MHC class I molecules within the same phagosome or on the cell surface (vacuolar
pathway) (2). Alternatively, antigens may escape from the phagosome and enter the cytosol (phagosome-to-
cytosol pathway) (3) to be processed via the classical MHC class I pathway. It has been suggested recently that
elements of the ER can be associated with phagosomes, allowing transfer of antigens into the cytosol by the
ER-associated degradation pathway and degradation by the phagosome-associated proteasome (3a). The
importance of each pathway (2, 3) for cross-presentation in vivo as well as the precise mechanisms and the
locations of antigen processing in each model are under investigation. TAP, Transporter associated with antigen
processing.