Page 536 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 536
Chapter 33 Pathobiology of the Human Erythrocyte and Its Hemoglobins 451
Hb variants with: saturated). It therefore has an extremely high oxygen affinity and
oxygen affinity would not be useful for delivering oxygen to tissues. The oxygen in
pH myoglobin is passed on to the mitochondria, where oxidative metabo-
100 BPG, PCO , or Temp lism occurs. The sigmoidal shape of the oxygen dissociation curve of
2
Hb indicates that the totally deoxygenated Hb tetramer is slow to
become oxygenated, but as oxygenation proceeds, the reaction of
Methemoglobin
heme with oxygen accelerates. Perutz has drawn an analogy in which
the “appetite” of heme for oxygen grows with the “eating,” and
80 conversely, loss of oxygen by heme lowers the oxygen affinity of the
Hb variants with: remaining heme groups. The Hill coefficient, n, which can be calcu-
oxygen affinity lated from plots of oxygen equilibrium curves, is a description of
pH heme–heme interaction or cooperativity that explains in part the
BPG, PCO , or Temp oxygen-binding properties of Hb and myoglobin. The Hill coefficient
60 2 for myoglobin is 1, indicating no cooperativity; n is approximately 3
% saturation for the normal human HbA molecule.
The oxygen affinity of Hb within the erythrocyte does not depend
solely on the intrinsic properties of the tetramer. The position of the
40
Hb oxygen dissociation curve, and therefore the P 50 , can be influenced
by a number of heterotropic modifiers, including temperature, pH,
and small organic phosphate molecules in the cell. The effects of these
modifiers on P 50 are shown in Fig. 33.3.
Hb is the prototype of an allosteric protein; its structure and
20 function are influenced by other molecules. The major intracellular
modulator of Hb–oxygen affinity in human erythrocytes is 2,3-BPG,
an intermediate product of glycolysis that is present within the
erythrocyte at concentrations equimolar to Hb. The synthesis of
0 2,3-BPG is enzymatically regulated, and its levels can change depend-
0 20 40 60 80 100 ing on the conditions extant. 2,3-BPG is able to bind stereospecifi-
cally within the central cavity of the Hb tetramer. Hb prepared in
Oxygen tension (mm Hg)-Po 2 the absence of 2,3-BPG has a very high oxygen affinity, but as
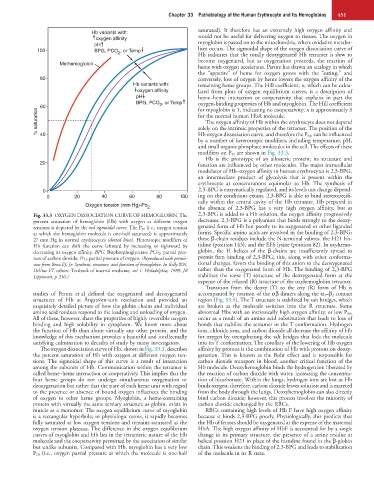
Fig. 33.3 OXYGEN DISSOCIATION CURVE OF HEMOGLOBIN. The 2,3-BPG is added to a Hb solution, the oxygen affinity progressively
percent saturation of hemoglobin (Hb) with oxygen at different oxygen decreases. 2,3-BPG is a polyanion that binds strongly to the deoxy-
tensions is depicted by the red sigmoidal curve. The P 50 (i.e., oxygen tension genated form of Hb but poorly to its oxygenated or other liganded
at which the hemoglobin molecule is one-half saturated) is approximately forms. Specific amino acids are involved in the binding of 2,3-BPG;
27 mm Hg in normal erythrocytes (dotted lines). Heterotopic modifiers of these β-chain residues include the N-terminal valines, the H21 his-
Hb function can shift the curve leftward by increasing or rightward by tidine (position 143), and the EF6 lysine (position 82). In oxyhemo-
decreasing its oxygen affinity. BPG, Bisphosphoglycerate PCO 2 , partial pres- globin, the H helices of the β-chains are insufficiently spread to
sure of carbon dioxide; PO 2 , partial pressure of oxygen. (Reproduced with permis- permit firm binding of 2,3-BPG; this, along with other conforma-
sion from Benz EJ, Jr: Synthesis, structure, and function of hemoglobin. In Kelly WN, tional changes, favors the binding of this anion to the deoxygenated
DeVita VT, editors: Textbook of internal medicine, vol 1. Philadelphia, 1989, JB rather than the oxygenated form of Hb. The binding of 2,3-BPG
Lippincott, p 236.) stabilizes the tense (T) structure of the deoxygenated form at the
expense of the relaxed (R) structure of the oxyhemoglobin tetramer.
Transition from the deoxy (T) to the oxy (R) form of Hb is
studies of Perutz et al defined the oxygenated and deoxygenated accompanied by rotation of the αβ dimers along the α 1 –β 2 contact
structures of Hb at Ångström-unit resolution and provided an region (Fig. 33.5). The T structure is stabilized by salt bridges, which
exquisitely detailed picture of how the globin chains and individual are broken as the molecule switches into the R structure. Some
amino acid residues respond to the loading and unloading of oxygen. abnormal Hbs with an intrinsically high oxygen affinity, or low P 50 ,
All of these, however, share the properties of highly reversible oxygen occur as a result of an amino acid substitution that leads to loss of
binding and high solubility in cytoplasm. We know more about bonds that stabilize the tetramer in the T conformation. Hydrogen
the function of Hb than about virtually any other protein, and the ions, chloride ions, and carbon dioxide all decrease the affinity of Hb
knowledge of this mechanism provides a beautiful and intellectually for oxygen by strengthening the salt bridges that lock the molecule
satisfying culmination to decades of study by many investigators. into its T conformation. The corollary of the lowering of Hb oxygen
The oxygen dissociation curve of Hb, shown in Fig. 33.3, describes affinity by protons is the combination of Hb with protons on deoxy-
the percent saturation of Hb with oxygen at different oxygen ten- genation. This is known as the Bohr effect and is responsible for
sions. The sigmoidal shape of this curve is a result of interaction carbon dioxide transport in blood, another critical function of the
among the subunits of Hb. Communication within the tetramer is Hb molecule. Deoxyhemoglobin binds the hydrogen ion liberated by
called heme–heme interaction or cooperativity. This implies that the the reaction of carbon dioxide with water, increasing the concentra-
four heme groups do not undergo simultaneous oxygenation or tion of bicarbonate. Within the lungs, hydrogen ions are lost as Hb
deoxygenation but rather that the state of each heme unit with regard binds oxygen; therefore, carbon dioxide leaves solution and is excreted
to the presence or absence of bound oxygen influences the binding from the body through the lungs. Deoxyhemoglobin can also directly
of oxygen to other heme groups. Myoglobin, a heme-containing bind carbon dioxide; however, this process involves the minority of
protein with virtually the same tertiary structure as globin, exists in carbon dioxide exchanged by the RBCs.
muscle as a monomer. The oxygen equilibrium curve of myoglobin RBCs containing high levels of Hb F have high oxygen affinity
is a rectangular hyperbola; in physiologic terms, it rapidly becomes because it binds 2,3-BPG poorly. Physiologically, this predicts that
fully saturated at low oxygen tensions and remains saturated as the the Hb of fetuses should be oxygenated at the expense of the maternal
oxygen tension plateaus. The difference in the oxygen equilibrium HbA. The high oxygen affinity of HbF is accounted for by a single
curves of myoglobin and Hb lies in the tetrameric nature of the Hb change in its primary structure, the presence of a serine residue at
molecule and the cooperativity permitted by the association of similar helical position H21 in place of the histidine found in the β-globin
but unlike subunits. Compared with Hb, myoglobin has a very low chain. This weakens the binding of 2,3-BPG and leads to stabilization
P 50 (i.e., oxygen partial pressure at which the molecule is one-half of the molecule in its R state.