Page 537 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 537
452 Part V Red Blood Cells
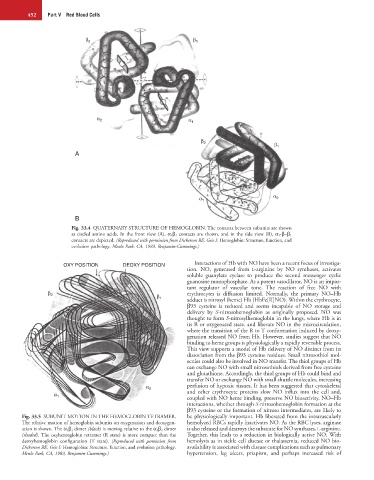
β 2 β 1
α 2 α 1
β 2 β 1
A
α 1 α 2
B
Fig. 33.4 QUATERNARY STRUCTURE OF HEMOGLOBIN. The contacts between subunits are shown
as circled amino acids. In the front view (A), α 1 β 2 contacts are shown, and in the side view (B), α 1 -β–β 1
contacts are depicted. (Reproduced with permission from Dickerson RE, Geis I: Hemoglobin: Structure, function, and
evolution pathology. Menlo Park, CA, 1983, Benjamin-Cummings.)
OXY POSITION DEOXY POSITION Interactions of Hb with NO have been a recent focus of investiga-
tion. NO, generated from L-arginine by NO synthases, activates
soluble guanylate cyclase to produce the second messenger cyclic
guanosine monophosphate. As a potent vasodilator, NO is an impor-
tant regulator of vascular tone. The reaction of free NO with
β 2 β 1 erythrocytes is diffusion limited. Normally, the primary NO–Hb
adduct is nitrosyl (heme) Hb (HbFe[II]NO). Within the erythrocyte,
β93 cysteine is reduced and seems incapable of NO storage and
delivery by S-nitrosohemoglobin as originally proposed. NO was
thought to form S-nitrosylhemoglobin in the lungs, where Hb is in
its R or oxygenated state, and liberate NO in the microcirculation,
where the transition of the R to T conformation induced by deoxy-
genation released NO from Hb. However, studies suggest that NO
binding to heme groups is physiologically a rapidly reversible process.
This view supports a model of Hb delivery of NO distinct from its
dissociation from the β93 cysteine residues. Small nitrosothiol mol-
ecules could also be involved in NO transfer. The thiol groups of Hb
can exchange NO with small nitrosothiols derived from free cysteine
and glutathione. Accordingly, the thiol groups of Hb could bind and
transfer NO or exchange NO with small shuttle molecules, increasing
α 2 perfusion of hypoxic tissues. It has been suggested that cytoskeletal
α 1 and other erythrocyte proteins slow NO influx into the cell and,
coupled with NO heme binding, preserve NO bioactivity. NO–Hb
interactions, whether through S-nitrosohemoglobin formation at the
β93 cysteine or the formation of nitroso intermediates, are likely to
Fig. 33.5 SUBUNIT MOTION IN THE HEMOGLOBIN TETRAMER. be physiologically important. Hb liberated from the intravascularly
The relative motion of hemoglobin subunits on oxygenation and deoxygen- hemolyzed RBCs rapidly inactivates NO. As the RBC lyses, arginase
ation is shown. The α 1 β 1 dimer (black) is moving relative to the α 2 β 2 dimer is also released and destroys the substrate for NO synthases, L-arginine.
(shaded). The oxyhemoglobin tetramer (R state) is more compact than the Together, this leads to a reduction in biologically active NO. With
deoxyhemoglobin configuration (T state). (Reproduced with permission from hemolysis as in sickle cell disease or thalassemia, reduced NO bio-
Dickerson RE, Geis I: Hemoglobin: Structure, function, and evolution pathology. availability is associated with disease complications such as pulmonary
Menlo Park, CA, 1983, Benjamin-Cummings.) hypertension, leg ulcers, priapism, and perhaps increased risk of