Page 852 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 852
Chapter 52 Histiocytic Disorders 735
C
A B D D E F
G H I J
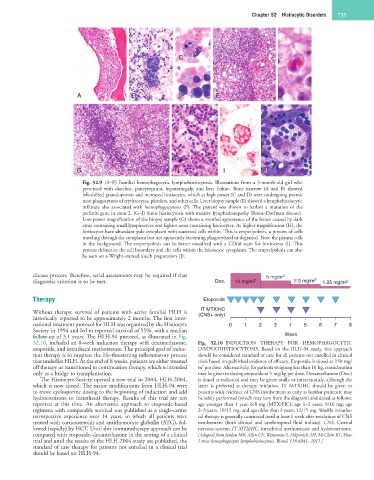
Fig. 52.9 (A–F) Familial hemophagocytic lymphohistiocytosis. Illustrations from a 3-month-old girl who
presented with diarrhea, pancytopenia, hepatomegaly, and liver failure. Bone marrow (A and B) showed
left-shifted granulopoiesis and increased histiocytes, which at high power (C and D) were undergoing promi-
nent phagocytosis of erythrocytes, platelets, and other cells. Liver biopsy sample (E) showed a lymphohistiocytic
infiltrate also associated with hemophagocytosis (F). The patient was shown to harbor a mutation of the
perforin gene in exon 2. (G–J) Sinus histiocytosis with massive lymphadenopathy (Rosai–Dorfman disease).
Low-power magnification of the biopsy sample (G) shows a mottled appearance of the lesion caused by dark
areas containing small lymphocytes and lighter areas containing histiocytes. At higher magnification (H), the
histiocytes have abundant pale cytoplasm with scattered cells within. This is emperipolesis, a process of cells
traveling through the cytoplasm but not apparently becoming phagocytized or degraded. Note the plasma cells
in the background. The emperipolesis can be better visualized with a CD68 stain for histiocytes (I). This
process delineates the cell boundary and the cells within the histiocyte cytoplasm. The emperipolesis can also
be seen on a Wright-stained touch preparation (J).
disease process; therefore, serial assessments may be required if that 5 mg/m 2
diagnostic criterion is to be met. Dex. 10 mg/m 2 2.5 mg/m 2 1.25 mg/m 2
Therapy Etoposide
IT MTX/HC
Without therapy, survival of patients with active familial HLH is (CNS+ only)
historically reported to be approximately 2 months. The first inter-
national treatment protocol for HLH was organized by the Histiocyte 0 1 2 3 4 5 6 7 8
Society in 1994 and led to reported survival of 55%, with a median
follow-up of 3.1 years. The HLH-94 protocol, as illustrated in Fig. Week
52.10, included an 8-week induction therapy with dexamethasone, Fig. 52.10 INDUCTION THERAPY FOR HEMOPHAGOCYTIC
etoposide, and intrathecal methotrexate. The principal goal of induc- LYMPHOHISTIOCYTOSIS. Based on the HLH-94 study, this approach
tion therapy is to suppress the life-threatening inflammatory process should be considered standard of care for all patients not enrolled in clinical
that underlies HLH. At the end of 8 weeks, patients are either weaned trials based on published evidence of efficacy. Etoposide is dosed as 150 mg/
2
off therapy or transitioned to continuation therapy, which is intended m per dose. Alternatively, for patients weighing less than 10 kg, consideration
only as a bridge to transplantation. may be given to dosing etoposide as 5 mg/kg per dose. Dexamethasone (Dex.)
The Histiocyte Society opened a new trial in 2004, HLH-2004, is dosed as indicated and may be given orally or intravenously, although the
which is now closed. The major modifications from HLH-94 were latter is preferred at therapy initiation. IT MTX/HC should be given to
to move cyclosporine dosing to the beginning of induction and add patients with evidence of CNS involvement as early as lumbar puncture may
hydrocortisone to intrathecal therapy. Results of this trial are not be safely performed (which may vary from the diagram) and dosed as follows:
reported at this time. An alternative approach to etoposide-based age younger than 1 year, 6/8 mg (MTX/HC); age 1–2 years, 8/10 mg; age
regimens with comparable survival was published as a single-center 2–3 years, 10/12 mg; and age older than 3 years, 12/15 mg. Weekly intrathe-
retrospective experience over 14 years, in which all patients were cal therapy is generally continued until at least 1 week after resolution of CNS
treated with corticosteroids and antithymocyte globulin (ATG), fol- involvement (both clinical and cerebrospinal fluid indices). CNS, Central
lowed (rapidly) by HCT. Until this immunotherapy approach can be nervous system; IT MTX/HC, intrathecal methotrexate and hydrocortisone.
compared with etoposide–dexamethasone in the setting of a clinical (Adapted from Jordan MB, Allen CE, Weitzman S, Filipovich AH, McClain KL: How
trial and until the results of the HLH-2004 study are published, the I treat hemophagocytic lymphohistiocytosis. Blood 118:4041, 2011.)
standard of care therapy for patients not enrolled in a clinical trial
should be based on HLH-94.