Page 848 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 848
Chapter 52 Histiocytic Disorders 731
In addition to late malignancies, patients should be monitored for
signs of long-term disabilities, including cosmetic, orthopedic, and
cutaneous deformities that may lead to loss of function and emotional
disorders, loss of permanent dentition, endocrinologic disorders and
growth failure, hearing impairment, CNS abnormalities and neuro-
cognitive function, sclerosing cholangitis with biliary cirrhosis, and
pulmonary fibrosis and cor pulmonale.
Future Directions
The treatment of LCH has undergone significant refinement over
the past decade. However, therapy remains empirically defined.
With the recent insights into the pathophysiology of LCH, such as
B-Raf mutations, new possibilities for more intelligently designed,
targeted therapies are conceivable. Trials are ongoing for the assess-
ment of B-Raf and Mek inhibitors in pediatric solid malignancies.
It is expected that pilot trials in patients with LCH will commence
in the near future. Future clinical trials for such targeted thera-
pies will likely focus on patients with therapy-resistant risk organ
involvement because these children have the least satisfactory
outcomes.
JUVENILE XANTHOGRANULOMATOUS DISEASE
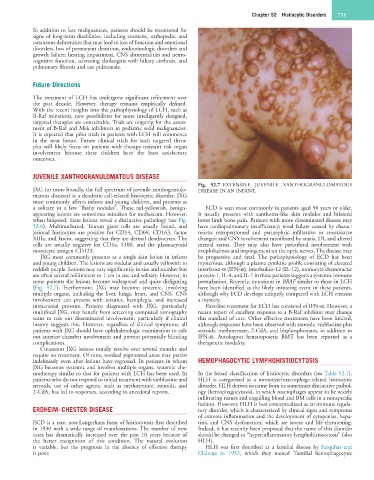
Fig. 52.7 EXTENSIVE JUVENILE XANTHOGRANULOMATOUS
JXG (or more broadly, the full spectrum of juvenile xanthogranulo- DISEASE IN AN INFANT.
matous diseases) is a dendritic cell-related histiocytic disorder. JXG
most commonly affects infants and young children, and presents as
a solitary or a few “fleshy nodules”. These red-yellowish, benign- ECD is seen most commonly in patients aged 50 years or older.
appearing lesions are sometimes mistaken for molluscum. However, It usually presents with xanthoma-like skin nodules and bilateral
when biopsied, these lesions reveal a distinctive pathology (see Fig. lower limb bone pain. Patients with more disseminated disease may
52.6). Multinucleated, Touton giant cells are usually found, and have cardiopulmonary insufficiency; renal failure caused by charac-
lesional histiocytes are positive for CD14, CD68, CD163, factor teristic retroperitoneal and perinephric infiltrative or constrictive
XIIIa, and fascin, suggesting that they are dermal dendrocytes. The changes; and CNS involvement manifested by ataxia, DI, and altered
cells are usually negative for CD1a, S100, and the plasmacytoid mental status. They may also have periorbital involvement with
monocyte antigen CD123. exophthalmos and impingement on the optic nerves. The disease may
JXG most commonly presents as a single skin lesion in infants be progressive and fatal. The pathophysiology of ECD has been
and young children. The lesions are nodular and usually yellowish to mysterious, although a plasma cytokine profile consisting of elevated
reddish purple. Lesions may vary significantly in size and number but interferon-α (IFN-α), interleukin-12 (IL-12), monocyte chemotactic
are often several millimeters to 1 cm in size and solitary. However, in protein-1, IL-4, and IL-7 in these patients suggests a systemic immune
some patients the lesions become widespread and quite disfiguring perturbation. Recently, mutations in BRAF similar to those in LCH
(Fig. 52.7). Furthermore, JXG may become systemic, involving have been identified as the likely initiating event in these patients,
multiple organs, including the liver, lungs, heart, and CNS. CNS although why ECD develops uniquely compared with LCH remains
involvement can present with seizures, hemiplegia, and increased a mystery.
intracranial pressure. Patients diagnosed with JXG, particularly First-line treatment for ECD has consisted of IFN-α. However, a
multifocal JXG, may benefit from screening computed tomography recent report of excellent response to a B-Raf inhibitor may change
scans to rule out disseminated involvement, particularly if clinical this standard of care. Other effective treatments have been limited,
history suggests this. However, regardless of clinical symptoms, all although responses have been observed with steroids, vinblastine plus
patients with JXG should have ophthalmologic examination to rule steroids, methotrexate, 2-CdA, and bisphosphonates, in addition to
out anterior chamber involvement and prevent potentially blinding IFN-α. Autologous hematopoietic BMT has been reported as a
complications. therapeutic modality.
Cutaneous JXG lesions usually resolve over several months and
require no treatment. Of note, residual pigmented areas may persist
indefinitely even after lesions have regressed. In patients in whom HEMOPHAGOCYTIC LYMPHOHISTIOCYTOSIS
JXG becomes systemic and involves multiple organs, systemic che-
motherapy similar to that for patients with LCH has been used. In In the broad classification of histiocytic disorders (see Table 52.1),
patients who do not respond to initial treatment with vinblastine and HLH is categorized as a monocyte/macrophage-related histiocytic
steroids, use of other agents, such as methotrexate, steroids, and disorder. HLH derives its name from its sometimes distinctive pathol-
2-CdA, has led to responses, according to anecdotal reports. ogy (hemophagocytosis), in which macrophages appear to be widely
infiltrating tissues and engulfing blood and BM cells in a nonspecific
fashion. However, HLH is best conceptualized as an immune regula-
ERDHEIM–CHESTER DISEASE tory disorder, which is characterized by clinical signs and symptoms
of extreme inflammation and the development of cytopenias, hepa-
ECD is a rare, non-Langerhans form of histiocytosis first described titis, and CNS dysfunction, which are severe and life threatening.
in 1930 with a wide range of manifestations. The number of new Indeed, it has recently been proposed that the name of this disorder
cases has dramatically increased over the past 10 years because of should be changed to “hyperinflammatory lymphohistiocytosis” (also
the better recognition of this condition. The natural evolution HLH).
is variable, but the prognosis in the absence of effective therapy HLH was first described as a familial disease by Farquhar and
is poor. Claireux in 1952, which they named “familial hemophagocytic