Page 862 - Hematology_ Basic Principles and Practice ( PDFDrive )
P. 862
Chapter 53 Lysosomal Storage Diseases: Perspectives and Principles 745
Sea-blue histiocyte
Sea-blue histiocyte
NPD foam cell
NPD foam cell
A B
C D
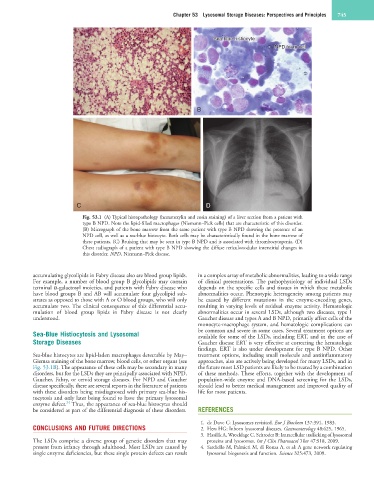
Fig. 53.1 (A) Typical histopathology (hematoxylin and eosin staining) of a liver section from a patient with
type B NPD. Note the lipid-filled macrophages (Niemann–Pick cells) that are characteristic of this disorder.
(B) Micrograph of the bone marrow from the same patient with type B NPD showing the presence of an
NPD cell, as well as a sea-blue histocyte. Both cells may be characteristically found in the bone marrow of
these patients. (C) Bruising that may be seen in type B NPD and is associated with thrombocytopenia. (D)
Chest radiograph of a patient with type B NPD showing the diffuse reticulonodular interstitial changes in
this disorder. NPD, Niemann–Pick disease.
accumulating glycolipids in Fabry disease also are blood group lipids. in a complex array of metabolic abnormalities, leading to a wide range
For example, a number of blood group B glycolipids may contain of clinical presentations. The pathophysiology of individual LSDs
terminal α-galactosyl moieties, and patients with Fabry disease who depends on the specific cells and tissues in which these metabolic
have blood groups B and AB will accumulate four glycolipid sub- abnormalities occur. Phenotypic heterogeneity among patients may
strates as opposed to those with A or O blood groups, who will only be caused by different mutations in the enzyme-encoding genes,
accumulate two. The clinical consequence of this differential accu- resulting in varying levels of residual enzyme activity. Hematologic
mulation of blood group lipids in Fabry disease is not clearly abnormalities occur in several LSDs, although two diseases, type 1
understood. Gaucher disease and types A and B NPD, primarily affect cells of the
monocyte-macrophage system, and hematologic complications can
Sea-Blue Histiocytosis and Lysosomal be common and severe in some cases. Several treatment options are
available for some of the LSDs, including ERT, and in the case of
Storage Diseases Gaucher disease ERT is very effective at correcting the hematologic
findings. ERT is also under development for type B NPD. Other
Sea-blue histocytes are lipid-laden macrophages detectable by May– treatment options, including small molecule and antiinflammatory
Giemsa staining of the bone marrow, blood cells, or other organs (see approaches, also are actively being developed for many LSDs, and in
Fig. 53.1B). The appearance of these cells may be secondary in many the future most LSD patients are likely to be treated by a combination
disorders, but for the LSDs they are principally associated with NPD, of these methods. These efforts, together with the development of
Gaucher, Fabry, or ceroid storage diseases. For NPD and Gaucher population-wide enzyme and DNA-based screening for the LSDs,
disease specifically, there are several reports in the literature of patients should lead to better medical management and improved quality of
with these disorders being misdiagnosed with primary sea-blue his- life for most patients.
tocytosis and only later being found to have the primary lysosomal
34
enzyme defect. Thus, the appearance of sea-blue histocytes should
be considered as part of the differential diagnosis of these disorders. REFERENCES
1. de Duve C: Lysosomes revisited. Eur J Biochem 137:391, 1983.
CONCLUSIONS AND FUTURE DIRECTIONS 2. Hers HG: Inborn lysosomal diseases. Gastroenterology 48:625, 1965.
3. Hasilik A, Wrocklage C, Schroder B: Intracellular trafficking of lysosomal
The LSDs comprise a diverse group of genetic disorders that may proteins and lysosomes. Int J Clin Pharmacol Ther 47:S18, 2009.
present from infancy through adulthood. Most LSDs are caused by 4. Sardiello M, Palmieri M, di Ronza A, et al: A gene network regulating
single enzyme deficiencies, but these single protein defects can result lysosomal biogenesis and function. Science 325:473, 2009.