Page 137 - Williams Hematology ( PDFDrive )
P. 137
112 Part III: Epochal Hematology Chapter 7: Hematology of the Fetus and Newborn 113
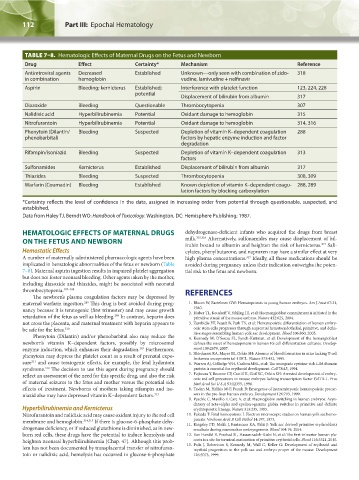
TABLE 7–8. Hematologic Effects of Maternal Drugs on the Fetus and Newborn
Drug Effect Certainty* Mechanism Reference
Antiretroviral agents Decreased Established Unknown—only seen with combination of zido- 318
in combination hemoglobin vudine, lamivudine + nelfinavir
Aspirin Bleeding; kernicterus Established; Interference with platelet function 123, 224, 228
potential
Displacement of bilirubin from albumin 317
Diazoxide Bleeding Questionable Thrombocytopenia 307
Nalidixic acid Hyperbilirubinemia Potential Oxidant damage to hemoglobin 315
Nitrofurantoin Hyperbilirubinemia Potential Oxidant damage to hemoglobin 314, 316
Phenytoin (Dilantin/ Bleeding Suspected Depletion of vitamin K–dependent coagulation 288
phenobarbital) factors by hepatic enzyme induction and factor
degradation
Rifampin/isoniazid Bleeding Suspected Depletion of vitamin K–dependent coagulation 313
factors
Sulfonamides Kernicterus Established Displacement of bilirubin from albumin 317
Thiazides Bleeding Suspected Thrombocytopenia 308, 309
Warfarin (Coumadin) Bleeding Established Known depletion of vitamin K–dependent coagu- 288, 289
lation factors by blocking carboxylation
*Certainty reflects the level of confidence in the data, assigned in increasing order from potential through questionable, suspected, and
established.
Data from Haley TJ, Berndt WO: Handbook of Toxicology. Washington, DC: Hemisphere Publishing; 1987.
HEMATOLOGIC EFFECTS OF MATERNAL DRUGS dehydrogenase-deficient infants who acquired the drugs from breast
ON THE FETUS AND NEWBORN milk. 315,316 Alternatively, sulfonamides may cause displacement of bil-
317
irubin bound to albumin and heighten the risk of kernicterus. Sali-
Hemostatic Effects cylates, phenylbutazone, and naproxen may have a similar effect at very
A number of maternally administered pharmacologic agents have been high plasma concentrations. Ideally, all these medications should be
317
implicated in hematologic abnormalities of the fetus or newborn (Table avoided during pregnancy unless their indication outweighs the poten-
7–8). Maternal aspirin ingestion results in impaired platelet aggregation tial risk to the fetus and newborn.
but does not foster neonatal bleeding. Other agents taken by the mother,
including diazoxide and thiazides, might be associated with neonatal
thrombocytopenia. 307–309 REFERENCES
The newborn’s plasma coagulation factors may be depressed by
maternal warfarin ingestion. This drug is best avoided during preg- 1. Bloom W, Bartelmez GW: Hematopoiesis in young human embryos. Am J Anat 67:21,
289
nancy because it is teratogenic (first trimester) and may cause growth 1940.
retardation of the fetus as well as bleeding. In contrast, heparin does 2. Huber TL, Kouskoff V, Fehling HJ, et al: Haemangioblast commitment is initiated in the
289
primitive streak of the mouse embryo. Nature 432:625, 2004.
not cross the placenta, and maternal treatment with heparin appears to 3. Zambidis ET, Peault B, Park TS, et al: Hematopoietic differentiation of human embry-
be safe for the fetus. 310 onic stem cells progresses through sequential hematoendothelial, primitive, and defin-
itive stages resembling human yolk sac development. Blood 106:860, 2005.
Phenytoin (Dilantin) and/or phenobarbital also may reduce the 4. Kennedy M, D’Souza SL, Lynch-Kattman, et al: Development of the hemangioblast
newborn’s vitamin K–dependent factors, possibly by microsomal defines the onset of hematopoiesis in human ES cell differentiation cultures. Develop-
enzyme induction, which enhances their degradation. Furthermore, ment 109:2679, 2007.
287
phenytoin may depress the platelet count as a result of prenatal expo- 5. Shivdasani RA, Mayer EL, Orkin SH: Absence of blood formation in mice lacking T-cell
leukemia oncoprotein tal-1/SCL. Nature 373:432, 1995.
sure and cause teratogenic effects, for example, the fetal hydantoin 6. Warren AJ, Colledge WH, Carlton MBL, et al: The oncogenic cysteine-rich LIM domain
311
syndrome. The decision to use this agent during pregnancy should protein is essential for erythroid development. Cell 78:45, 1994.
312
reflect an assessment of the need for this specific drug, and also the risk 7. Fujiwara Y, Browne CP, Cuniff K, Goff SC, Orkin SH: Arrested development of embry-
of maternal seizures to the fetus and mother versus the potential side onic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc
Natl Acad Sci U S A 93:12355, 1996.
effects of treatment. Newborns of mothers taking rifampin and iso- 8. Tavian M, Hallais M-F, Peault B: Emergence of intraembryonic hematopoietic precur-
niazid also may have depressed vitamin K–dependent factors. 313 sors in the pre-liver human embryo. Development 126:793, 1999.
9. Peschle C, Mavilio F, Care A, et al: Haemoglobin switching in human embryos: Asyn-
chrony of zeta→alpha and epsilon→gamma-globin switches in primitive and definite
Hyperbilirubinemia and Kernicterus erythropoietic lineage. Nature 313:235, 1985.
Nitrofurantoin and nalidixic acid may cause oxidant injury to the red cell 10. Fukuda T: Fetal hemopoiesis. I. Electron microscopic studies on human yolk sac hemo-
membrane and hemoglobin. 314,315 If there is glucose-6-phosphate dehy- poiesis. Virchows Arch B Cell Pathol 14:197, 1973.
drogenase deficiency, or if reduced glutathione is diminished, as in new- 11. Kingsley PD, Malik J, Fantauzzo KA, Palis J: Yolk sac derived primitive erythroblasts
enucleate during mammalian embryogenesis. Blood 104:19, 2004.
born red cells, these drugs have the potential to induce hemolysis and 12. Van Handel B, Prashad SL, Hassanzadeh-Kiabi N, et al: The first trimester human pla-
heighten neonatal hyperbilirubinemia (Chap. 47). Although this prob- centa is a site for terminal maturation of primitive erythroid cells. Blood 116:3321, 2010.
lem has not been documented by transplacental transfer of nitrofuran- 13. Palis J, Robertson S, Kennedy M, Wall C, Keller G: Development of erythroid and
myeloid progenitors in the yolk sac and embryo proper of the mouse. Development
toin or nalidixic acid, hemolysis has occurred in glucose-6-phosphate 126:5073, 1999.
Kaushansky_chapter 07_p0097-0118.indd 112 9/18/15 10:13 PM