Page 1670 - Williams Hematology ( PDFDrive )
P. 1670
1644 Part XI: Malignant Lymphoid Diseases Chapter 99: Follicular Lymphoma 1645
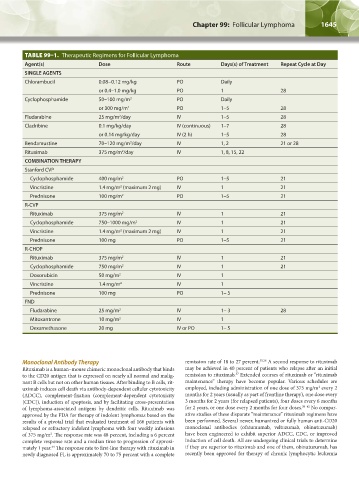
TABLE 99–1. Therapeutic Regimens for Follicular Lymphoma
Agent(s) Dose Route Days(s) of Treatment Repeat Cycle at Day
SINGLE AGENTS
Chlorambucil 0.08−0.12 mg/kg PO Daily
or 0.4–1.0 mg/kg PO 1 28
Cyclophosphamide 50–100 mg/m 2 PO Daily
or 300 mg/m 2 PO 1–5 28
Fludarabine 25 mg/m /day IV 1–5 28
2
Cladribine 0.1 mg/kg/day IV (continuous) 1–7 28
or 0.14 mg/kg/day IV (2 h) 1–5 28
Bendamustine 70–120 mg/m /day IV 1, 2 21 or 28
2
Rituximab 375 mg/m /day IV 1, 8, 15, 22
2
COMBINATION THERAPY
Stanford CVP
Cyclophosphamide 400 mg/m 2 PO 1–5 21
Vincristine 1.4 mg/m (maximum 2 mg) IV 1 21
2
Prednisone 100 mg/m 2 PO 1–5 21
R-CVP
Rituximab 375 mg/m 2 IV 1 21
Cyclophosphamide 750–1000 mg/m 2 IV 1 21
Vincristine 1.4 mg/m (maximum 2 mg) IV 1 21
2
Prednisone 100 mg PO 1–5 21
R-CHOP
Rituximab 375 mg/m 2 IV 1 21
Cyclophosphamide 750 mg/m 2 IV 1 21
Doxorubicin 50 mg/m 2 IV 1
Vincristine 1.4 mg/m 2 IV 1
Prednisone 100 mg PO 1– 5
FND
Fludarabine 25 mg/m 2 IV 1– 3 28
Mitoxantrone 10 mg/m 2 IV 1
Dexamethasone 20 mg IV or PO 1– 5
Monoclonal Antibody Therapy remission rate of 18 to 27 percent. 35,36 A second response to rituximab
Rituximab is a human–mouse chimeric monoclonal antibody that binds may be achieved in 40 percent of patients who relapse after an initial
37
to the CD20 antigen that is expressed on nearly all normal and malig- remission to rituximab. Extended courses of rituximab or “rituximab
nant B cells but not on other human tissues. After binding to B cells, rit- maintenance” therapy have become popular. Various schedules are
2
uximab induces cell death via antibody-dependent cellular cytotoxicity employed, including administration of one dose of 375 mg/m every 2
(ADCC), complement-fixation (complement-dependent cytotoxicity months for 2 years (usually as part of frontline therapy), one dose every
[CDC]), induction of apoptosis, and by facilitating cross-presentation 3 months for 2 years (for relapsed patients), four doses every 6 months
of lymphoma-associated antigens by dendritic cells. Rituximab was for 2 years, or one dose every 2 months for four doses. 38–42 No compar-
approved by the FDA for therapy of indolent lymphomas based on the ative studies of these disparate “maintenance” rituximab regimens have
results of a pivotal trial that evaluated treatment of 166 patients with been performed. Several newer, humanized or fully human anti-CD20
relapsed or refractory indolent lymphoma with four weekly infusions monoclonal antibodies (ofatumumab, veltuzumab, obinutuzumab)
of 375 mg/m . The response rate was 48 percent, including a 6 percent have been engineered to exhibit superior ADCC, CDC, or improved
2
complete response rate and a median time to progression of approxi- induction of cell death. All are undergoing clinical trials to determine
mately 1 year. The response rate to first-line therapy with rituximab in if they are superior to rituximab and one of them, obinutuzumab, has
34
newly diagnosed FL is approximately 70 to 75 percent with a complete recently been approved for therapy of chronic lymphocytic leukemia
Kaushansky_chapter 99_p1641-1652.indd 1645 9/18/15 3:57 PM