Page 1671 - Williams Hematology ( PDFDrive )
P. 1671
1646 Part XI: Malignant Lymphoid Diseases Chapter 99: Follicular Lymphoma 1647
TABLE 99–2. Selected Randomized Studies of Chemotherapy Alone Versus Rituximab Plus Chemotherapy for First-Line
Therapy of Follicular Lymphoma
Treatment, No. of Median Followup Median TTP/TTF/EFS
Study Patients (Months) ORR (%) CR (%) (Months) OS (%)
Marcus 45 CVP, 159 53 57 10 15 77
R-CVP, 162 81 41 34 83
p <0.0001 p = 0.0290
Hiddemann 46 CHOP, 205 18 90 17 29 74
R-CHOP, 223 96 20 NR 87
p <0.001 p = 0.016
Herold 47 MCP, 96 47 75 25 26 74
R-MCP, 105 92 50 NR 87
p <0.0001 p = 0.0096
Bachy 43 CHVP-IFN, 183 100 73 63 34 69
R-CHVP-IFN, 175 84 79 66 78 at 8 years
p = 0.0004 p = 0.076
Hochster 44 CVP, 158 36 82 22 16 92
CVP + rituximab 86 37 59 86 at 3 years
maintenance, 153
P = 4.4 × 10 −10 p = 0.05 one sided
CR, complete response; CHOP, cyclophosphamide, doxorubicin, vincristine, prednisone; CHVP, cyclophosphamide, doxorubicin, teniposide,
prednisone; CVP, cyclophosphamide, vincristine, prednisone; EFS, event-free survival; IFN, interferon; MCP, mitoxantrone, chlorambucil, predni-
sone; ORR, overall response rate; OS, overall survival; R, rituximab; TTF, time to treatment failure; TTP, time to progression.
(CLL) (but not FL). “Biosimilar” CD20 antibodies will also soon be cyclophosphamide, vincristine, and prednisone (R-CVP) was com-
available as an alternative to rituximab. pared to eight cycles of CVP without rituximab in 321 patients with
newly diagnosed FL (Fig. 99–4). R-CVP was superior to CVP alone
48
Rituximab Plus Chemotherapy in terms of ORR (81 percent vs. 57 percent), CR rate (41 percent vs.
The introduction of rituximab into treatment protocols for FL has 10 percent), time to progression (34 months vs. 15 months), time to
revolutionized the management of this disease. Multiple randomized, treatment failure (27 months vs. 7 months), and OS (83 percent vs.
controlled clinical trials have documented the superiority of combin- 77 percent at 4 years, p = 0.029). Similarly, R-CHOP was compared to
45
ing rituximab with chemotherapy compared to the use of chemother- CHOP for first-line treatment of 428 patients with advanced stage FL.
apy alone in terms of overall response rates (ORRs), complete response R-CHOP exhibited a superior ORR (96 percent vs. 90 percent), time
(CR) rates, event-free survival (EFS), PFS, and OS (Table 99–2). 43–47 In to treatment failure (p <0.001), duration of response (p = 0.001), and
one study, induction therapy consisting of eight cycles of rituximab, OS (p = 0.016) compared to CHOP alone. Similar benefits have also
46
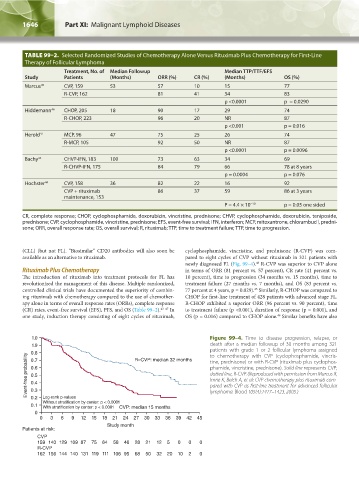
1.0 Figure 99–4. Time to disease progression, relapse, or
1.9 death after a median followup of 30 months among 321
patients with grade 1 or 2 follicular lymphoma assigned
0.8 R–CVP: median 32 months to chemotherapy with CVP (cyclophosphamide, vincris-
Event-free probability 0.6 phamide, vincristine, prednisone). Solid line represents CVP;
0.7
tine, prednisone) or with R-CVP (rituximab plus cyclophos-
dotted line, R-CVP. (Reproduced with permission from Marcus R,
0.5
Imrie K, Belch A, et al: CVP chemotherapy plus rituximab com-
0.4
pared with CVP as first-line treatment for advanced follicular
0.3
Log-rank p-values
0.2
Without stratification by center: p < 0.0001 lymphoma. Blood 105(4):1417–1423, 2005.)
0.1 With stratification by center: p < 0.0001 CVP: median 15 months
0
0 3 6 9 12 15 18 21 24 27 30 33 36 39 42 45
Study month
Patients at risk:
CVP
159 140 129 109 87 75 64 58 46 28 21 12 5 0 0 0
R-CVP
162 156 144 140 131 119 111 106 95 68 50 32 20 10 2 0
Kaushansky_chapter 99_p1641-1652.indd 1646 9/18/15 3:57 PM