Page 1805 - Williams Hematology ( PDFDrive )
P. 1805
1780 Part XI: Malignant Lymphoid Diseases Chapter 108: Immunoglobulin Light-Chain Amyloidosis 1781
A B
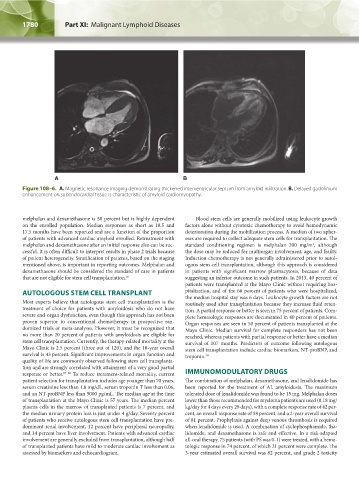
Figure 108–6. A. Magnetic resonance imaging demonstrating thickened interventricular septum from amyloid infiltration. B. Delayed gadolinium
enhancement on subendocardial tissue is characteristic of amyloid cardiomyopathy.
melphalan and dexamethasone is 50 percent but is highly dependent Blood stem cells are generally mobilized using leukocyte growth
on the enrolled population. Median responses as short as 10.5 and factors alone without cytotoxic chemotherapy to avoid hemodynamic
17.5 months have been reported and are a function of the proportion deterioration during the mobilization process. A median of two apher-
of patients with advanced cardiac amyloid enrolled. Retreatment with eses are required to collect adequate stem cells for transplantation. The
melphalan and dexamethasone after an initial response also can be suc- standard conditioning regimen is melphalan 200 mg/m , although
2
cessful. It is often difficult to interpret results in phase 2 trials because the dose may be reduced for multiorgan involvement, age, and frailty.
of patient heterogeneity. Stratification of patients, based on the staging Induction chemotherapy is not generally administered prior to autol-
mentioned above, is important in reporting outcomes. Melphalan and ogous stem cell transplantation, although this approach is considered
dexamethasone should be considered the standard of care in patients in patients with significant marrow plasmacytosis, because of data
that are not eligible for stem cell transplantation. 66 suggesting an inferior outcome in such patients. In 2013, 40 percent of
patients were transplanted at the Mayo Clinic without requiring hos-
AUTOLOGOUS STEM CELL TRANSPLANT pitalization, and of the 60 percent of patients who were hospitalized,
the median hospital stay was 6 days. Leukocyte growth factors are not
Most experts believe that autologous stem cell transplantation is the routinely used after transplantation because they increase fluid reten-
treatment of choice for patients with amyloidosis who do not have tion. A partial response or better is seen in 75 percent of patients. Com-
severe end-organ dysfunction, even though this approach has not been plete hematologic responses are documented in 40 percent of patients.
proven superior to conventional chemotherapy in prospective ran- Organ responses are seen in 50 percent of patients transplanted at the
domized trials or meta-analyses. However, it must be recognized that Mayo Clinic. Median survival for complete responders has not been
no more than 20 percent of patients with amyloidosis are eligible for reached, whereas patients with partial response or better have a median
stem cell transplantation. Currently, the therapy-related mortality at the survival of 107 months. Predictors of outcome following autologous
Mayo Clinic is 2.5 percent (three out of 120), and the 10-year overall stem cell transplantation include cardiac biomarkers, NT-proBNP, and
survival is 43 percent. Significant improvements in organ function and troponin. 70
quality of life are commonly observed following stem cell transplanta-
tion and are strongly correlated with attainment of a very good partial
response or better. 67–69 To reduce treatment-related mortality, current IMMUNOMODULATORY DRUGS
patient selection for transplantation includes age younger than 70 years, The combination of melphalan, dexamethasone, and lenalidomide has
serum creatinine less than 1.8 mg/dL, serum troponin T less than 0.06, been reported for the treatment of AL amyloidosis. The maximum
and an NT-proBNP less than 5000 pg/mL. The median age at the time tolerated dose of lenalidomide was found to be 15 mg. Melphalan doses
of transplantation at the Mayo Clinic is 57 years. The median percent lower than those recommended for myeloma patients are used (0.18 mg/
plasma cells in the marrow of transplanted patients is 7 percent, and kg/day for 4 days every 28 days), with a complete response rate of 42 per-
the median urinary protein loss is just under 4 g/day. Seventy percent cent, an overall response rate of 58 percent, and a 2-year overall survival
of patients who receive autologous stem cell transplantation have pre- of 81 percent. Prophylaxis against deep venous thrombosis is required
dominant renal involvement, 12 percent have peripheral neuropathy, when lenalidomide is used. A combination of cyclophosphamide, tha-
and 14 percent have liver involvement. Patients with advanced cardiac lidomide, and dexamethasone is safe and effective. In a risk-adapted
involvement are generally excluded from transplantation, although half all-oral therapy, 75 patients (with PS was 0-1) were treated, with a hema-
of transplanted patients have mild to moderate cardiac involvement as tologic response in 74 percent, of which 21 percent were complete. The
assessed by biomarkers and echocardiogram. 3-year estimated overall survival was 82 percent, and grade 2 toxicity
Kaushansky_chapter 108_p1773-1784.indd 1780 9/18/15 9:53 AM