Page 1811 - Williams Hematology ( PDFDrive )
P. 1811
1786 Part XI: Malignant Lymphoid Diseases Chapter 109: Macroglobulinemia 1787
A B
C D
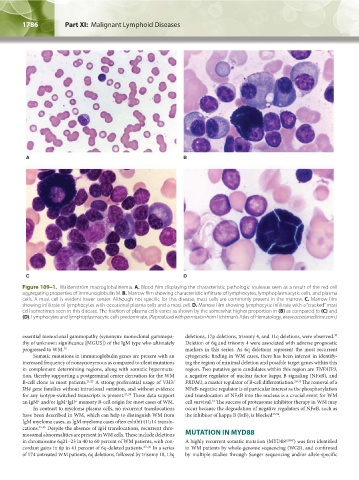
Figure 109–1. Waldenström macroglobulinemia. A. Blood film displaying the characteristic pathologic rouleaux seen as a result of the red cell
aggregating properties of immunoglobulin M. B. Marrow film showing characteristic infiltrate of lymphocytes, lymphoplasmacytic cells, and plasma
cells. A mast cell is evident lower center. Although not specific for this disease, mast cells are commonly present in the marrow. C. Marrow film
showing infiltrate of lymphocytes with occasional plasma cells and a mast cell. D. Marrow film showing lymphocytic infiltrate with a “cracked” mast
cell sometimes seen in this disease. The fraction of plasma cells varies as shown by the somewhat higher proportion in (B) as compared to (C) and
(D). Lymphocytes and lymphoplasmacytic cells predominate. (Reproduced with permission from Lichtman’s Atlas of Hematology, www.accessmedicine.com.)
30
essential monoclonal gammopathy (synonym: monoclonal gammopa- deletions, 17p deletions, trisomy 4, and 11q deletions, were observed.
thy of unknown significance [MGUS]) of the IgM type who ultimately Deletion of 6q and trisomy 4 were associated with adverse prognostic
progressed to WM. 20 markers in this series. As 6q deletions represent the most recurrent
Somatic mutations in immunoglobulin genes are present with an cytogenetic finding in WM cases, there has been interest in identify-
increased frequency of nonsynonymous as compared to silent mutations ing the region of minimal deletion and possible target genes within this
in complement determining regions, along with somatic hypermuta- region. Two putative gene candidates within this region are TNFAIP3,
tion, thereby supporting a postgerminal center derivation for the WM a negative regulator of nuclear factor kappa B signaling (NFκB), and
B-cell clone in most patients. 21,22 A strong preferential usage of VH3/ PRDM1, a master regulator of B-cell differentiation. 29,31 The removal of a
JH4 gene families without intraclonal variation, and without evidence NFκB-negative regulator is of particular interest as the phosphorylation
for any isotype-switched transcripts is present. 23,24 These data support and translocation of NFκB into the nucleus is a crucial event for WM
+
an IgM and/or IgM IgD memory B-cell origin for most cases of WM. cell survival. The success of proteasome inhibitor therapy in WM may
32
+
+
In contrast to myeloma plasma cells, no recurrent translocations occur because the degradation of negative regulators of NFκB, such as
have been described in WM, which can help to distinguish WM from the inhibitor of kappa B (IκB), is blocked 33,34 .
IgM myeloma cases, as IgM myeloma cases often exhibit t11;14 translo-
cations. 25,26 Despite the absence of IgH translocations, recurrent chro-
mosomal abnormalities are present in WM cells. These include deletions MUTATION IN MYD88
in chromosome 6q21–23 in 40 to 60 percent of WM patients, with con- A highly recurrent somatic mutation (MYD88 L265P ) was first identified
cordant gains in 6p in 41 percent of 6q-deleted patients. 27–30 In a series in WM patients by whole-genome sequencing (WGS), and confirmed
of 174 untreated WM patients, 6q deletions, followed by trisomy 18, 13q by multiple studies through Sanger sequencing and/or allele-specific
Kaushansky_chapter 109_p1785-1802.indd 1786 9/21/15 12:30 PM