Page 1862 - Williams Hematology ( PDFDrive )
P. 1862
1836 Part XII: Hemostasis and Thrombosis Chapter 112: Platelet Morphology, Biochemistry, and Function 1837
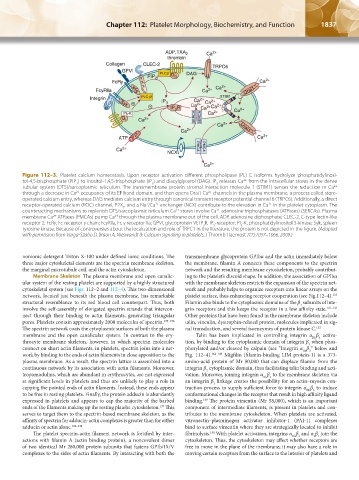
Figure 112–3. Platelet calcium homeostasis. Upon receptor activation different phospholipase (PL) C isoforms hydrolyze phosphatidylinosi-
2+
tol-4,5-bisphosphate (PIP ) to inositol-1,4,5-trisphosphate (IP ) and diacylglycerol (DAG). IP releases Ca from the intracellular stores in the dense
3
2
3
2+
tubular system (DTS)/sarcoplasmic reticulum. The transmembrane protein stromal interaction molecule 1 (STIM1) senses the reduction in Ca
2+
through a decrease in Ca occupancy of its EF hand domain, and then opens Orai1 Ca channels in the plasma membrane, a process called store-
2+
operated calcium entry, whereas DAG mediates calcium entry through canonical transient receptor potential channel 6 (TRPC6). Additionally, a direct
2+
2+
receptor-operated calcium (ROC) channel, P2X , and a Na /Ca exchanger (NCX) contribute to the elevation in Ca in the platelet cytoplasm. The
+
1
2+
counteracting mechanisms to replenish DTS/sarcoplasmic reticulum Ca stores involve Ca adenosine triphosphatases (ATPases) (SERCAs). Plasma
2+
2+
membrane Ca ATPases (PMCAs) pump Ca through the plasma membrane out of the cell. ADP, adenosine diphosphate; CLEC-2, C-type lectin-like
2+
receptor 2; FcRγ, Fc receptor γ chain; FcγRIIa, Fc γ receptor IIa; GPVI, glycoprotein VI; IP R, IP -receptor; PI -K, phosphatidylinositol 3-kinase; Syk, spleen
3
3
3
tyrosine kinase. Because of controversies about the localization and role of TRPC1 in the literature, this protein is not depicted in the figure. (Adapted
with permission from Varga-Szabo D, Braun A, Nieswandt B: Calcium signaling in platelets. J Thromb Haemost 7(7):1057–1066, 2009.)
nonionic detergent Triton X-100 under defined ionic conditions. The transmembrane glycoprotein GPIbα and the actin immediately below
three major cytoskeletal elements are the spectrin membrane skeleton, the membrane, filamin A connects these components to the spectrin
the marginal microtubule coil, and the actin cytoskeleton. network and the resulting membrane cytoskeleton, probably contribut-
Membrane Skeleton The plasma membrane and open canalic- ing to the platelet’s discoid shape. In addition, the association of GPIbα
ular system of the resting platelet are supported by a highly structured with the membrane skeleton restricts the expansion of the spectrin net-
cytoskeletal system (see Figs. 112–2 and 112–4). This two-dimensional work and probably helps to organize receptors into linear arrays on the
133
network, located just beneath the plasma membrane, has remarkable platelet surface, thus enhancing receptor cooperation (see Fig.112–4).
structural resemblance to its red blood cell counterpart. Thus, both Filamin also binds to the cytoplasmic domains of the β subunits of inte-
3
involve the self-assembly of elongated spectrin strands that intercon- grin receptors and this keeps the receptor in a low affinity state. 141–143
nect through their binding to actin filaments, generating triangular Other proteins that have been found in the membrane skeleton include
pores. Platelets contain approximately 2000 molecules of spectrin. 133–136 talin, vinculin, dystrophin-related protein, molecules implicated in sig-
The spectrin network coats the cytoplasmic surfaces of both the plasma nal transduction, and several isoenzymes of protein kinase C. 133
membrane and the open canalicular system. In contrast to the ery- Talin has been implicated in controlling integrin α β activa-
IIb 3
throcyte membrane skeleton, however, in which spectrin molecules tion, by binding to the cytoplasmic domain of integrin β when phos-
3
connect on short actin filaments, in platelets, spectrin joins into a net- phorylated and/or cleaved by calpain (see “Integrin α β ” below and
IIb 3
work by binding to the ends of actin filaments in close apposition to the Fig. 112–4). 144–148 Migfilin (filamin-binding LIM protein-1) is a 373-
plasma membrane. As a result, the spectrin lattice is assembled into a amino-acid protein of Mr 50,000 that can displace filamin from the
continuous network by its association with actin filaments. Moreover, integrin β cytoplasmic domain, thus facilitating talin binding and acti-
3
tropomodulins, which are abundant in erythrocytes, are not expressed vation. Moreover, joining integrin α β to the membrane skeleton via
IIb 3
at significant levels in platelets and thus are unlikely to play a role in an integrin β linkage creates the possibility for an actin–myosin con-
3
capping the pointed ends of actin filaments. Instead, these ends appear traction process to supply sufficient force to integrin α β to induce
IIb 3
to be free in resting platelets. Finally, the protein adducin is abundantly conformational changes in the receptor that result in high affinity ligand
expressed in platelets and appears to cap the majority of the barbed binding. The protein vimentin (Mr 58,000), which is an important
149
137
ends of the filaments making up the resting platelet cytoskeleton. This component of intermediate filaments, is present in platelets and con-
serves to target them to the spectrin-based membrane skeleton, as the tributes to the membrane cytoskeleton. When platelets are activated,
affinity of spectrin for adducin-actin complexes is greater than for either vitronectin–plasminogen activator inhibitor-1 (PAI-1) complexes
adducin or actin alone. 138–140 bind to surface vimentin where they are strategically located to inhibit
150
The platelet spectrin-actin filament network is fortified by inter- fibrinolysis. With platelet activation, integrins α β and α β join the
IIb 3
2 1
actions with filamin A (actin binding protein), a noncovalent dimer cytoskeleton. Thus, the cytoskeleton may affect whether receptors are
of two identical Mr 280,000 protein subunits that fastens GPIb/IX/V free to move in the plane of the membrane; it may also have a role in
complexes to the sides of actin filaments. By interacting with both the moving certain receptors from the surface to the interior of platelets and
Kaushansky_chapter 112_p1829-1914.indd 1837 17/09/15 3:26 pm