Page 2056 - Williams Hematology ( PDFDrive )
P. 2056
2030 Part XII: Hemostasis and Thrombosis Chapter 118: Heparin-induced Thrombocytopenia 2031
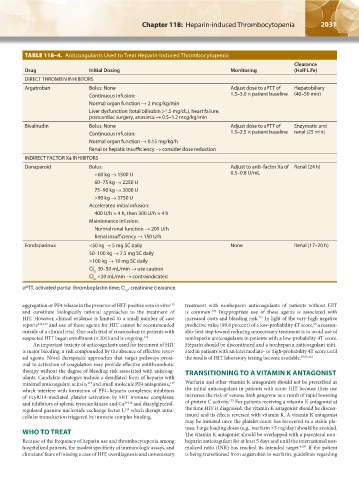
TABLE 118–4. Anticoagulants Used to Treat Heparin-Induced Thrombocytopenia
Clearance
Drug Initial Dosing Monitoring (Half-Life)
DIRECT THROMBIN INHIBITORS
Argatroban Bolus: None Adjust dose to aPTT of Hepatobiliary
Continuous infusion: 1.5–3.0 × patient baseline (40–50 min)
Normal organ function → 2 mcg/kg/min
Liver dysfunction (total bilirubin >1.5 mg/dL), heart failure,
postcardiac surgery, anasarca → 0.5–1.2 mcg/kg/min
Bivalirudin Bolus: None Adjust dose to aPTT of Enzymatic and
Continuous infusion: 1.5–2.5 × patient baseline renal (25 min)
Normal organ function → 0.15 mg/kg/h
Renal or hepatic insufficiency → consider dose reduction
INDIRECT FACTOR Xa INHIBITORS
Danaparoid Bolus: Adjust to anti–factor Xa of Renal (24 h)
<60 kg → 1500 U 0.5–0.8 U/mL
60–75 kg → 2250 U
75–90 kg → 3000 U
>90 kg → 3750 U
Accelerated initial infusion:
400 U/h × 4 h, then 300 U/h × 4 h
Maintenance infusion:
Normal renal function → 200 U/h
Renal insufficiency → 150 U/h
Fondaparinux <50 kg → 5 mg SC daily None Renal (17–20 h)
50–100 kg → 7.5 mg SC daily
>100 kg → 10 mg SC daily
Cl 30–50 mL/min → use caution
Cr
Cl <30 mL/min → contraindicated
Cr
aPTT, activated partial thromboplastin time; Cl , creatinine clearance.
Cr
aggregation or PF4 release in the presence of HIT-positive sera in vitro treatment with nonheparin anticoagulants of patients without HIT
113
120
and constitute biologically rational approaches to the treatment of is common. Inappropriate use of these agents is associated with
121
HIT. However, clinical evidence is limited to a small number of case increased costs and bleeding risk. In light of the very-high negative
65
reports 114,115 and use of these agents for HIT cannot be recommended predictive value (99.8 percent) of a low-probability 4T score, a reason-
outside of a clinical trial. One such trial of rivaroxaban in patients with able first step toward reducing unnecessary treatment is to avoid use of
suspected HIT began enrollment in 2013 and is ongoing. 116 nonheparin anticoagulants in patients with a low-probability 4T score.
An important toxicity of anticoagulants used for treatment of HIT Heparin should be discontinued and a nonheparin anticoagulant initi-
is major bleeding, a risk compounded by the absence of effective rever- ated in patients with an intermediate- or high-probability 4T score until
sal agents. Novel therapeutic approaches that target pathways proxi- the results of HIT laboratory testing become available. 65,95,122
mal to activation of coagulation may provide effective antithrombotic
therapy without the degree of bleeding risk associated with anticoag- TRANSITIONING TO A VITAMIN K ANTAGONIST
ulants. Candidate strategies include a desulfated form of heparin with
117
minimal anticoagulant activity and small molecule PF4 antagonists, Warfarin and other vitamin K antagonists should not be prescribed as
113
which interfere with formation of PF4–heparin complexes; inhibitors the initial anticoagulant in patients with acute HIT because their use
of FcγRIIA-mediated platelet activation by HIT immune complexes; increases the risk of venous limb gangrene as a result of rapid lowering
123
and inhibitors of splenic tyrosine kinase and Ca 2+118 and diacylglycerol- of protein C activity. For patients receiving a vitamin K antagonist at
regulated guanine nucleotide exchange factor I, which disrupt intra- the time HIT is diagnosed, the vitamin K antagonist should be discon-
119
cellular transduction triggered by immune complex binding. tinued and its effects reversed with vitamin K. A vitamin K antagonist
may be initiated once the platelet count has recovered to a stable pla-
teau. Large loading doses (e.g., warfarin >5 mg/day) should be avoided.
WHO TO TREAT The vitamin K antagonist should be overlapped with a parenteral non-
Because of the frequency of heparin use and thrombocytopenia among heparin anticoagulant for at least 5 days and until the international nor-
hospitalized patients, the modest specificity of immunologic assays, and malized ratio (INR) has reached its intended target. 63,95 If the patient
clinicians’ fears of missing a case of HIT, overdiagnosis and unnecessary is being transitioned from argatroban to warfarin, guidelines regarding
Kaushansky_chapter 118_p2025-2034.indd 2031 9/18/15 5:43 PM