Page 2068 - Williams Hematology ( PDFDrive )
P. 2068
2042 Part XII: Hemostasis and Thrombosis Chapter 120: Hereditary Qualitative Platelet Disorders 2043
abnormalities of platelet integrin α (GPIIb; CD41) and/or integrin β Jordan, and Saudi Arabia; 30 patients from Italy; a smaller number of
IIb
3
(GPIIIa; CD61). 16 patients from three Gypsy families; and 43 patients from Pakistan. 22,32–40
In 1918, Eduard Glanzmann, a Swiss pediatrician, described a Perhaps the highest frequency of a GT mutation is found in the Iraqi-
group of patients with hemorrhagic symptoms and a defect in platelet Jewish population where the most common mutation causing GT was
function, namely the ability to retract clots (“weak” platelets or throm- found in six of 700 individuals. 39
17
basthenia). Subsequent studies demonstrated that thrombasthenic The platelet integrin α β receptor is required for platelet aggrega-
IIb 3
patients have prolonged bleeding times and that their platelets fail to tion induced by all physiologic agonists (ADP, epinephrine, thrombin,
41
aggregate in response to physiologic agonists 18–21 and have markedly collagen, TXA ) (Chap. 112). Consequently, abnormalities in the
2
23
reduced 18,20–22 platelet fibrinogen. In the mid-1970s, Nurden and Caen receptor result in a failure of platelet plug formation at sites of vascular
24
and Phillips and colleagues discovered that thrombasthenic platelets injury, and excessive bleeding.
are deficient in both integrin α and β . Later studies demonstrated that The integrin α β receptor is also responsible for the uptake of
IIb 3
3
IIb
integrin α and β form a calcium-dependent complex in the platelet fibrinogen from plasma into α granules, hence, patients with GT have
42
IIb
3
membrane that functions as a receptor for fibrinogen and other adhe- markedly reduced platelet fibrinogen. 18,20,21,43,44 Clot retraction requires
sive glycoproteins. 25–28 Cloning and sequencing of the complementary platelets with intact integrin α β receptors, 45,46 and is, therefore, usu-
IIb 3
DNAs for integrin α IIb 29 and β identified them as separate protein ally abnormal in GT. 18
30
3
subunits that are members of the integrin receptor superfamily and Defects in either integrin α or β result in the same functional
31
IIb
3
permitted the molecular biological characterization of patients with the defect because both subunits are required for receptor function (Chap. 112).
disorder (see database of Glanzmann patients http://med.mssm.edu/ Biosynthetic studies indicate that integrin α and β form a com-
IIb
3
glanzmanndb). plex soon after protein synthesis in the rough endoplasmic reticu-
50
lum 47–49 ; subsequent posttranslational processing and transport to the
Etiology and Pathogenesis platelet membrane require that the complex be intact (Fig. 120–2). 51,52
GT is a rare disorder characterized by autosomal recessive inheritance Complex formation protects each of the glycoproteins from proteolytic
with a worldwide distribution. In regions where consanguineous mat- digestion, 47–50 so if either integrin α or β is absent or unable to form a
3
IIb
ings are common, groups of patients with the disorder have been identi- normal complex, the other subunit will be rapidly degraded, most likely
fied, and in several populations founder mutations have been identified through a proteasomal mechanism. Thus, a deficiency in either glyco-
by analyzing polymorphisms in the DNA surrounding the affected protein produces a deficiency in both. Because complex formation and
mutation. These include 42 patients from South India; 39 patients from vesicular transport are also required for proteolytic processing of pro-
50
the Iraqi-Jewish population in Israel; 46 Arab patients from Israel, α into its constituent α α and α β subunits, if these processes do
IIb
IIb
IIb
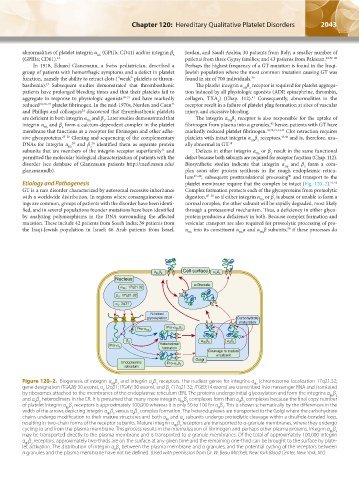
Figure 120–2. Biogenesis of integrin α β and integrin α β receptors. The nuclear genes for integrins α (chromosome localization 17q21.32;
IIb
IIb 3
V 3
gene designation ITGA2B; 30 exons), α (2q31; ITGAV; 30 exons), and β (17q21.32; ITGB3;14 exons) are transcribed into messenger RNA and translated
V
3
by ribosomes attached to the membranes of the endoplasmic reticulum (ER). The proteins undergo initial glycosylation and form the integrins α β
IIb 3
and α β heterodimers in the ER. It is presumed that many more integrin α β complexes form than α β complexes because the final copy number
V 3
V 3
IIb 3
of platelet integrin α β receptors is approximately 100,000 whereas it is only 50 to 100 for α β . This is shown schematically by the differences in the
IIb 3
V 3
width of the arrows depicting integrin α β versus α β complex formation. The heteroduplexes are transported to the Golgi where the carbohydrate
IIb 3
V 3
chains undergo modification to their mature structures and both α and α subunits undergo proteolytic cleavage within a disulfide-bonded loop,
V
IIb
resulting in two-chain forms of the receptor subunits. Mature integrin α β receptors are transported to α-granule membranes, where they undergo
IIb 3
cycling to and from the plasma membrane. This process results in the internalization of fibrinogen and perhaps other plasma proteins. Integrin α β
IIb 3
may be transported directly to the plasma membrane and is transported to α granule membranes. Of the total of approximately 100,000 integrin
α β receptors, approximately two-thirds are on the surface at any given time and the remaining one-third can be brought to the surface by plate-
IIb 3
let activation. The distribution of integrin α β between the plasma membrane and α granules, and the potential cycling of the receptors between
V 3
α granules and the plasma membrane have not be defined. (Used with permission from Dr. W. Beau Mitchell, New York Blood Center, New York, NY.)
Kaushansky_chapter 120_p2039-2072.indd 2043 9/21/15 2:20 PM