Page 207 - Williams Hematology ( PDFDrive )
P. 207
182 Part IV: Molecular and Cellular Hematology Chapter 13: Cytogenetics and Genetic Abnormalities 183
with a poor prognosis. With respect to epigenetic changes, transcrip- performed. 52,53 The ACMG recommends disclosure of genetic informa-
33
53
tional silencing via DNA methylation of the CDKN2B (p15 INK4B ) gene tion regarding 24 genes that confer germline cancer predisposition.
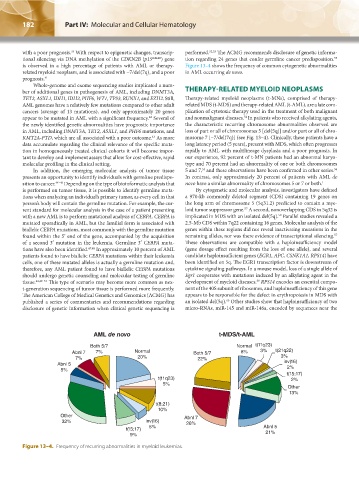
is observed in a high percentage of patients with AML or therapy- Figure 13–4 shows the frequency of common cytogenetic abnormalities
related myeloid neoplasm, and is associated with −7/del(7q), and a poor in AML occurring de novo.
prognosis. 41
Whole-genome and exome sequencing studies implicated a num-
ber of additional genes in pathogenesis of AML, including DNMT3A, THERAPY-RELATED MYELOID NEOPLASMS
TET2, ASXL1, IDH1, IDH2, PHF6, WT1, TP53, RUNX1, and EZH2. Still, Therapy-related myeloid neoplasms (t-MNs), comprised of therapy-
AML genomes have a relatively few mutations compared to other adult related MDS (t-MDS) and therapy-related AML (t-AML), are a late com-
cancers (average of 13 mutations), and only approximately 20 genes plication of cytotoxic therapy used in the treatment of both malignant
54
appear to be mutated in AML with a significant frequency. Several of and nonmalignant diseases. In patients who received alkylating agents,
42
the newly identified genetic abnormalities have prognostic importance the characteristic recurring chromosome abnormalities observed are
in AML, including DNMT3A, TET2, ASXL1, and PHF6 mutations, and loss of part or all of chromosomes 5 [del(5q)] and/or part or all of chro-
43
KMT2A-PTD, which are all associated with a poor outcome. As more mosome 7 [−7/del(7q)] (see Fig. 13–4). Clinically, these patients have a
data accumulate regarding the clinical relevance of the specific muta- long latency period (5 years), present with MDS, which often progresses
tion in homogeneously treated clinical cohorts it will become impor- rapidly to AML with multilineage dysplasia and a poor prognosis. In
tant to develop and implement assays that allow for cost-effective, rapid our experience, 92 percent of t-MN patients had an abnormal karyo-
molecular profiling in the clinical setting. type and 70 percent had an abnormality of one or both chromosomes
In addition, the emerging molecular analysis of tumor tissue 5 and 7, and these observations have been confirmed in other series.
56
55
presents an opportunity to identify individuals with germline predispo- In contrast, only approximately 20 percent of patients with AML de
sition to cancer. 44–46 Depending on the type of bioinformatic analysis that novo have a similar abnormality of chromosomes 5 or 7 or both. 1
is performed on tumor tissue, it is possible to identify germline muta- By cytogenetic and molecular analysis, investigators have defined
tions when analyzing an individual’s primary tumor, as every cell in that a 970-kb commonly deleted segment (CDS) containing 19 genes on
person’s body will contain the germline mutation. For example, the cur- the long arm of chromosome 5 (5q31.2) predicted to contain a mye-
57
rent standard for molecular analysis in the case of a patient presenting loid tumor suppressor gene. A second, nonoverlapping CDS in 5q32 is
58
with a new AML is to perform mutational analysis of CEBPA. CEBPA is implicated in MDS with an isolated del(5q). Parallel studies revealed a
mutated sporadically in AML, but the familial form is associated with 2.5-Mb CDS within 7q22 containing 16 genes. Molecular analysis of the
biallelic CEBPA mutations, most commonly with the germline mutation genes within these regions did not reveal inactivating mutations in the
found within the 5′ end of the gene, accompanied by the acquisition remaining alleles, nor was there evidence of transcriptional silencing.
57
of a second 3′ mutation in the leukemia. Germline 3′ CEBPA muta- These observations are compatible with a haploinsufficiency model
tions have also been identified. 47,48 In approximately 10 percent of AML (gene dosage effect resulting from the loss of one allele), and several
patients found to have biallelic CEBPA mutations within their leukemia candidate haploinsufficient genes (EGR1, APC, CSNK1A1, RPS14) have
cells, one of these mutated alleles is actually a germline mutation and, been identified on 5q. The EGR1 transcription factor is downstream of
therefore, any AML patient found to have biallelic CEBPA mutations cytokine signaling pathways. In a mouse model, loss of a single allele of
should undergo genetic counseling and molecular testing of germline Egr1 cooperates with mutations induced by an alkylating agent in the
59
tissue. 44,49–51 This type of scenario may become more common as nex- development of myeloid diseases. RPS14 encodes an essential compo-
t-generation sequencing of tumor tissue is performed more frequently. nent of the 40S subunit of ribosomes, and haploinsufficiency of this gene
The American College of Medical Genetics and Genomics (ACMG) has appears to be responsible for the defect in erythropoiesis in MDS with
60
published a series of commentaries and recommendations regarding an isolated del(5q). Other studies show that haploinsufficiency of two
disclosure of genetic information when clinical genetic sequencing is micro-RNAs, miR-145 and miR-146a, encoded by sequences near the
AML de novo t-MDS/t-AML
Both 5/7 Normal t(11q23)
Abnl 7 7% Normal Both 5/7 8% 3% t(21q22)
7% 20% 22% 3%
Abnl 5 inv(16)
5% 2%
t(15;17)
t(11q23) 2%
5%
Other
13%
t(8;21)
10%
Other Abnl 7
32% inv(16) 26%
t(15;17) 5% Abnl 5
9% 21%
Figure 13–4. Frequency of recurring abnormalities in myeloid leukemias.
Kaushansky_chapter 13_p0173-0190.indd 182 17/09/15 6:32 pm