Page 205 - Williams Hematology ( PDFDrive )
P. 205
180 Part IV: Molecular and Cellular Hematology Chapter 13: Cytogenetics and Genetic Abnormalities 181
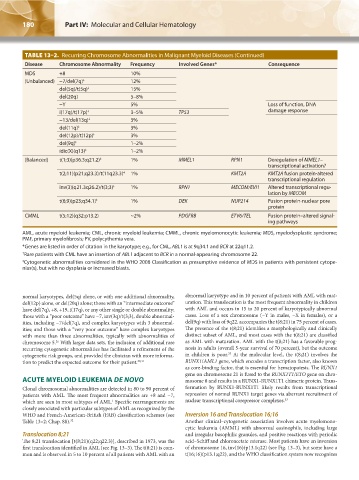
TABLE 13–2. Recurring Chromosome Abnormalities in Malignant Myeloid Diseases (Continued)
Disease Chromosome Abnormality Frequency Involved Genes* Consequence
MDS +8 10%
(Unbalanced) −7/del(7q) ‡ 12%
del(5q)/t(5q) ‡ 15%
del(20q) 5–8%
−Y 5% Loss of function, DNA
i(17q)/t(17p) ‡ 3–5% TP53 damage response
−13/del(13q) ‡ 3%
del(11q) ‡ 3%
del(12p)/t(12p) ‡ 3%
del(9q) ‡ 1–2%
idic(X)(q13) ‡ 1–2%
(Balanced) t(1;3)(p36.3;q21.2) ‡ 1% MMEL1 RPN1 Deregulation of MMEL1–
transcriptional activation?
t(2;11)(p21;q23.3)/t(11q23.3) 1% KMT2A KMT2A fusion protein-altered
‡
transcriptional regulation
inv(3)(q21.3q26.2)/t(3;3) 1% RPN1 MECOM/EVI1 Altered transcriptional regu-
‡
lation by MECOM
t(6;9)(p23;q34.1) ‡ 1% DEK NUP214 Fusion protein-nuclear pore
protein
CMML t(5;12)(q32;p13.2) ~2% PDGFRB ETV6/TEL Fusion protein–altered signal-
ing pathways
AML, acute myeloid leukemia; CML, chronic myeloid leukemia; CMML, chronic myelomonocytic leukemia; MDS, myelodysplastic syndrome;
PMF, primary myelofibrosis; PV, polycythemia vera.
*Genes are listed in order of citation in the karyotype; e.g., for CML, ABL1 is at 9q34.1 and BCR at 22q11.2.
† Rare patients with CML have an insertion of ABL1 adjacent to BCR in a normal-appearing chromosome 22.
‡ Cytogenetic abnormalities considered in the WHO 2008 Classification as presumptive evidence of MDS in patients with persistent cytope-
nias(s), but with no dysplasia or increased blasts.
normal karyotypes, del(5q) alone, or with one additional abnormality, abnormal karyotype and in 10 percent of patients with AML with mat-
del(12p) alone, or del(20q) alone; those with an “intermediate outcome” uration. This translocation is the most frequent abnormality in children
have del(7q), +8, +19, i(17q), or any other single or double abnormality; with AML and occurs in 15 to 20 percent of karyotypically abnormal
those with a “poor outcome” have −7, inv(3q)/t(3;3), double abnormal- cases. Loss of a sex chromosome (−Y in males, −X in females), or a
ities, including −7/del(7q), and complex karyotypes with 3 abnormal- del(9q) with loss of 9q22, accompanies the t(8;21) in 75 percent of cases.
ities; and those with a “very poor outcome” have complex karyotypes The presence of the t(8;21) identifies a morphologically and clinically
with more than three abnormalities, typically with abnormalities of distinct subset of AML, and most cases with the t(8;21) are classified
chromosome 5. With larger data sets, the inclusion of additional rare as AML with maturation. AML with the t(8;21) has a favorable prog-
26
recurring cytogenetic abnormalities has facilitated a refinement of the nosis in adults (overall 5-year survival of 70 percent), but the outcome
33
cytogenetic risk groups, and provided the clinician with more informa- in children is poor. At the molecular level, the t(8;21) involves the
tion to predict the expected outcome for their patient. 30,31 RUNX1/AML1 gene, which encodes a transcription factor, also known
as core-binding factor, that is essential for hematopoiesis. The RUNX1
gene on chromosome 21 is fused to the RUNX1T1/ETO gene on chro-
ACUTE MYELOID LEUKEMIA DE NOVO mosome 8 and results in a RUNX1-RUNX1T1 chimeric protein. Trans-
Clonal chromosomal abnormalities are detected in 80 to 90 percent of formation by RUNX1-RUNX1T1 likely results from transcriptional
patients with AML. The most frequent abnormalities are +8 and −7, repression of normal RUNX1 target genes via aberrant recruitment of
which are seen in most subtypes of AML. Specific rearrangements are nuclear transcriptional corepressor complexes. 33
1
closely associated with particular subtypes of AML as recognized by the
WHO and French-American-British (FAB) classification schemes (see Inversion 16 and Translocation 16;16
Table 13–2; Chap. 88). 32 Another clinical–cytogenetic association involves acute myelomono-
cytic leukemia (AMML) with abnormal eosinophils, including large
Translocation 8;21 and irregular basophilic granules, and positive reactions with periodic
The 8;21 translocation [t(8;21)(q22;q22.3)], described in 1973, was the acid–Schiff and chloroacetate esterase. Most patients have an inversion
first translocation identified in AML (see Fig. 13–3). The t(8;21) is com- of chromosome 16, inv(16)(p13.1q22) (see Fig. 13–3), but some have a
mon and is observed in 5 to 10 percent of all patients with AML with an t(16;16)(p13.1;q22), and the WHO classification system now recognizes
Kaushansky_chapter 13_p0173-0190.indd 180 17/09/15 6:32 pm