Page 2192 - Williams Hematology ( PDFDrive )
P. 2192
2166 Part XII: Hemostasis and Thrombosis Chapter 126: von Willebrand Disease 2167
Constitutive-like (basal) secretion
(? smaller VWF multimers)
(Constitutive secretion, pro-VWF)
Regulated secretion
Luminal (ultralarge VWF multimers)
?
?
Weibel-
palade
Golgi bodies
Endoplasmic
reticulum
Abluminal
Constitutive-like (basal) secretion
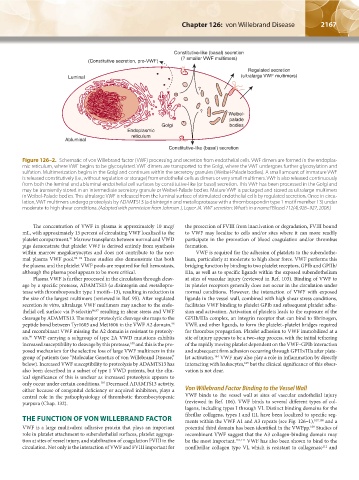
Figure 126–2. Schematic of von Willebrand factor (VWF) processing and secretion from endothelial cells. VWF dimers are formed in the endoplas-
mic reticulum, where VWF begins to be glycosylated. VWF dimers are transported to the Golgi, where the VWF undergoes further glycosylation and
sulfation. Multimerization begins in the Golgi and continues within the secretory granules (Weibel-Palade bodies). A small amount of immature VWF
is released constitutively (i.e., without regulation or storage) from endothelial cells as dimers or very small multimers. VWF is also released continuously
from both the luminal and abluminal endothelial cell surfaces by constitutive-like (or basal) secretion. This VWF has been processed in the Golgi and
may be transiently stored in an intermediate secretory granule or Weibel-Palade bodies. Mature VWF is packaged and stored as ultralarge multimers
in Weibel-Palade bodies. This ultralarge VWF is released from the luminal surface of stimulated endothelial cells by regulated secretion. Once in circu-
lation, VWF multimers undergo proteolysis by ADAMTS13 (a disintegrin and metalloprotease with a thrombospondin type 1 motif member 13) under
moderate to high shear conditions. (Adapted with permission from Johnsen J, Lopez JA. VWF secretion: What's in a name? Blood 112(4):926–927, 2008.)
The concentration of VWF in plasma is approximately 10 mcg/ the protection of FVIII from inactivation or degradation, FVIII bound
mL, with approximately 15 percent of circulating VWF localized to the to VWF may localize to cells and/or sites where it can more readily
platelet compartment. Marrow transplants between normal and VWD participate in the promotion of blood coagulation and/or thrombus
91
pigs demonstrate that platelet VWF is derived entirely from synthesis formation.
within marrow megakaryocytes and does not contribute to the nor- VWF is required for the adhesion of platelets to the subendothe-
mal plasma VWF pool. 92–94 These studies also demonstrate that both lium, particularly at moderate to high shear force. VWF performs this
the plasma and the platelet VWF pools are required for full hemostasis, bridging function by binding to two platelet receptors, GPIb and GPIIb/
although the plasma pool appears to be more critical. IIIa, as well as to specific ligands within the exposed subendothelium
Plasma VWF is further processed in the circulation through cleav- at sites of vascular injury (reviewed in Ref. 103). Binding of VWF to
age by a specific protease, ADAMTS13 (a disintegrin and metallopro- its platelet receptors generally does not occur in the circulation under
tease with thrombospondin type 1 motifs–13), resulting in reduction in normal conditions. However, the interaction of VWF with exposed
the size of the largest multimers (reviewed in Ref. 95). After regulated ligands in the vessel wall, combined with high shear stress conditions,
secretion in vitro, ultralarge VWF multimers may anchor to the endo- facilitates VWF binding to platelet GPIb and subsequent platelet adhe-
thelial cell surface via P-selectin 96,97 resulting in shear stress and VWF sion and activation. Activation of platelets leads to the exposure of the
cleavage by ADAMTS13. The major proteolytic cleavage site maps to the GPIIb/IIIa complex, an integrin receptor that can bind to fibrinogen,
peptide bond between Tyr1605 and Met1606 in the VWF A2 domain, VWF, and other ligands, to form the platelet–platelet bridges required
98
and recombinant VWF missing the A2 domain is resistant to proteoly- for thrombus propagation. Platelet adhesion to VWF immobilized at a
sis. VWF carrying a subgroup of type 2A VWD mutations exhibits site of injury appears to be a two-step process, with the initial tethering
99
increased susceptibility to cleavage by this protease, and this is the pro- of the rapidly moving platelet dependent on the VWF–GPIb interaction
100
posed mechanism for the selective loss of large VWF multimers in this and subsequent firm adhesion occurring through GPIIb/IIIa after plate-
group of patients (see “Molecular Genetics of von Willebrand Disease,” let activation. VWF may also play a role in inflammation by directly
104
below). Increased VWF susceptibility to proteolysis by ADAMTS13 has interacting with leukocytes, but the clinical significance of this obser-
105
also been described in a subset of type 1 VWD patients, but the clin- vation is not clear.
ical significance of this is unclear as increased proteolysis appears to
only occur under certain conditions. Decreased ADAMTS13 activity,
101
either because of congenital deficiency or acquired inhibitors, plays a Von Willebrand Factor Binding to the Vessel Wall
central role in the pathophysiology of thrombotic thrombocytopenic VWF binds to the vessel wall at sites of vascular endothelial injury
purpura (Chap. 132). (reviewed in Ref. 106). VWF binds to several different types of col-
lagens, including types I through VI. Distinct binding domains for the
fibrillar collagens, types I and III, have been localized to specific seg-
THE FUNCTION OF VON WILLEBRAND FACTOR ments within the VWF A1 and A3 repeats (see Fig. 126–1), 107,108 and a
VWF is a large multivalent adhesive protein that plays an important potential third domain has been identified in the VWFpp. Studies of
109
role in platelet attachment to subendothelial surfaces, platelet aggrega- recombinant VWF suggest that the A3 collagen-binding domain may
tion at sites of vessel injury, and stabilization of coagulation FVIII in the be the most important. 110,111 VWF has also been shown to bind to the
circulation. Not only is the interaction of VWF and FVIII important for nonfibrillar collagen type VI, which is resistant to collagenase and
112
Kaushansky_chapter 126_p2163-2182.indd 2167 9/21/15 3:14 PM