Page 229 - Williams Hematology ( PDFDrive )
P. 229
204 Part IV: Molecular and Cellular Hematology Chapter 15: Apoptosis Mechanisms: Relevance to the Hematopoietic System 205
From a functional perceptive, it is useful to view the caspases as either include cytoskeletal and nuclear matrix proteins, chromatin-modifying
upstream “initiator” caspases or downstream “effector” caspases. The (e.g., poly-ADP ribosyl polymerase [PARP]) and DNA repair proteins,
5
zymogen forms of upstream initiator caspases possess large N-terminal inhibitory subunits of endonucleases (CIDE-family proteins), protein
prodomains, which function as protein interaction modules, allowing kinases (often separating the autorepressing regulatory domains from
them to interact with various proteins that trigger caspase activation. In catalytic domains) and other signal transduction proteins.
contrast, the proforms of downstream effector caspases contain only short
N-terminal prodomains, serving no apparent function. Downstream cas- CASPASE ACTIVATION PATHWAYS
pases are largely dependent on upstream caspases for their proteolytic
processing and activation. The substrates of effector caspases are myriad, Several pathways for activating caspases have been delineated
as revealed in recent years by unbiased proteomics approaches. Substrates (Fig. 15–1). The simplest is exploited by cytolytic T lymphocytes (CTLs)
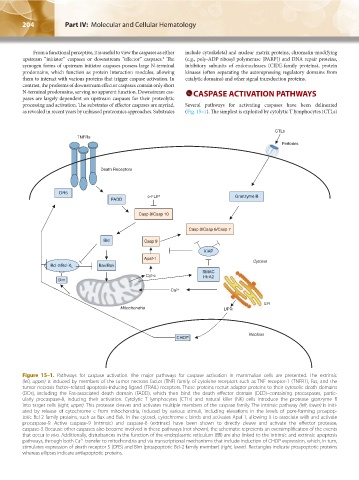
CTLs
TNFRs
Perforins
Death Receptors
DR5
c-FLIP Granzyme B
FADD
Casp 8/Casp 10
Casp 3/Casp 6/Casp 7
Bid Casp 9
XIAP
Apaf-1
Cytosol
Bcl-2/Bcl-X L Bax/Bak
SMAC
Cyt-c HtrA2
Bim
Ca 2+
ER
Mitochondria UPR
Nucleus
CHOP
Figure 15–1. Pathways for caspase activation. The major pathways for caspase activation in mammalian cells are presented. The extrinsic
(left, upper) is induced by members of the tumor necrosis factor (TNF) family of cytokine receptors such as TNF receptor-1 (TNFR1), Fas, and the
tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) receptors. These proteins recruit adapter proteins to their cytosolic death domains
(DDs), including the Fas-associated death domain (FADD), which then bind the death effector domain (DED)–containing procaspases, partic-
ularly procaspase-8, inducing their activation. Cytolytic T lymphocytes (CTLs) and natural killer (NK) cells introduce the protease granzyme B
into target cells (right, upper). This protease cleaves and activates multiple members of the caspase family. The intrinsic pathway (left, lower) is initi-
ated by release of cytochrome c from mitochondria, induced by various stimuli, including elevations in the levels of pore-forming proapop-
totic Bcl-2 family proteins, such as Bax and Bak. In the cytosol, cytochrome c binds and activates Apaf-1, allowing it to associate with and activate
procaspase-9. Active caspase-9 (intrinsic) and caspase-8 (extrinsic) have been shown to directly cleave and activate the effector protease,
caspase-3. Because other caspases also become involved in these pathways (not shown), the schematic represents an oversimplification of the events
that occur in vivo. Additionally, disturbances in the function of the endoplasmic reticulum (ER) are also linked to the intrinsic and extrinsic apoptosis
pathways, through both Ca transfer to mitochondria and via transcriptional mechanisms that include induction of CHOP expression, which, in turn,
2+
stimulates expression of death receptor 5 (DR5) and Bim (proapoptotic Bcl-2 family member) (right, lower). Rectangles indicate proapoptotic proteins
whereas ellipses indicate antiapoptotic proteins.
Kaushansky_chapter 15_p0203-0212.indd 204 17/09/15 6:37 pm