Page 224 - Williams Hematology ( PDFDrive )
P. 224
198 Part IV: Molecular and Cellular Hematology Chapter 14: Metabolism of Hematologic Neoplastic Cells 199
glutamine glucose
Warburg effect
pyruvate
lactate
acetyl-CoA
oxaloacetate
malate citrate
a ketoglutarate–dependent
fumarate isocitrate oxygenases:
IDH2, IDH3 DNA demethylation
succinate a ketoglutarate histone demethylation
glutamate
GLS isocitrate
glutamine
IDH1
mutant IDH1
glutaminolysis
NADPH NADP+
a ketoglutarate 2-hydroxyglutarate
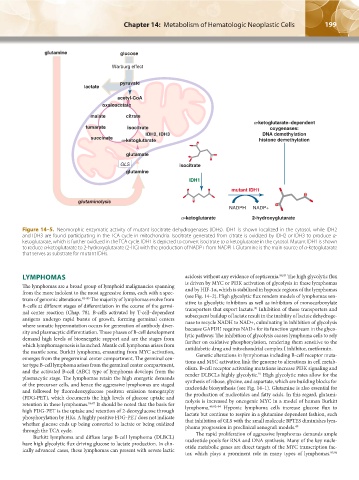
Figure 14–5. Neomorphic enzymatic activity of mutant isocitrate dehydrogenases (IDHs). IDH1 is shown localized in the cytosol, while IDH2
and IDH3 are found participating in the TCA cycle in mitochondria. Isocitrate generated from citrate is oxidized by IDH2 or IDH3 to produce α-
ketoglutarate, which is further oxidized in the TCA cycle. IDH1 is depicted to convert isocitrate to α-ketoglutarate in the cytosol. Mutant IDH1 is shown
to reduce α-ketoglutarate to 2-hydroxyglutarate (2-HG) with the production of NADP+ from NADPH. Glutamine is the main source of α-ketoglutarate
that serves as substrate for mutant IDHs.
LYMPHOMAS acidosis without any evidence of septicemia. 88,89 The high glycolytic flux
The lymphomas are a broad group of lymphoid malignancies spanning is driven by MYC or PI3K activation of glycolysis in these lymphomas
and by HIF-1α, which is stabilized in hypoxic regions of the lymphomas
from the more indolent to the most aggressive forms, each with a spec- (see Fig. 14–2). High glycolytic flux renders models of lymphomas sen-
trum of genomic alterations. 82–85 The majority of lymphomas evolve from sitive to glycolytic inhibitors as well as inhibitors of monocarboxylate
B-cells at different stages of differentiation in the course of the germi- transporters that export lactate. Inhibition of these transporters and
90
nal center reaction (Chap. 78). B-cells activated by T-cell–dependent subsequent buildup of lactate result in the inability of lactate dehydroge-
antigens undergo rapid bursts of growth, forming germinal centers nase to recycle NADH to NAD+, culminating in inhibition of glycolysis
where somatic hypermutation occurs for generation of antibody diver- because GAPDH requires NAD+ for its function upstream in the glyco-
sity and plasmacytic differentiation. These phases of B-cell development lytic pathway. The inhibition of glycolysis causes lymphoma cells to rely
demand high levels of bioenergetic support and are the stages from further on oxidative phosphorylation, rendering them sensitive to the
which lymphomagenesis is launched. Mantle cell lymphoma arises from antidiabetic drug and mitochondrial complex I inhibitor, metformin.
the mantle zone. Burkitt lymphoma, emanating from MYC activation, Genetic alterations in lymphomas including B-cell receptor muta-
emerges from the pregerminal center compartment. The germinal cen- tions and MYC activation link the genome to alterations in cell metab-
ter type B-cell lymphoma arises from the germinal center compartment, olism. B-cell receptor activating mutations increase PI3K signaling and
and the activated B-cell (ABC) type of lymphoma develops from the render DLBCLs highly glycolytic. High glycolytic rates allow for the
91
plasmacytic stage. The lymphomas retain the high energetic demands synthesis of ribose, glycine, and aspartate, which are building blocks for
of the precursor cells, and hence the aggressive lymphomas are staged nucleotide biosynthesis (see Fig. 14–1). Glutamine is also essential for
and followed by fluorodeoxyglucose positron emission tomography the production of nucleotides and fatty acids. In this regard, glutami-
(FDG-PET), which documents the high levels of glucose uptake and nolysis is increased by oncogenic MYC in a model of human Burkitt
retention in these lymphomas. 86,87 It should be noted that the basis for lymphoma. 49,92–94 Hypoxic lymphoma cells increase glucose flux to
high FDG-PET is the uptake and retention of 2-deoxyglucose through lactate but continue to respire in a glutamine dependent fashion, such
phosphorylation by HKs. A highly positive FDG-PET does not indicate that inhibition of GLS with the small molecule BPTES diminishes lym-
whether glucose ends up being converted to lactate or being oxidized phoma progression in preclinical xenograft models. 49
through the TCA cycle. The rapid proliferation of aggressive lymphomas demands ample
Burkitt lymphoma and diffuse large B-cell lymphoma (DLBCL) nucleotide pools for RNA and DNA synthesis. Many of the key nucle-
have high glycolytic flux driving glucose to lactate production. In clin- otide metabolic genes are direct targets of the MYC transcription fac-
ically advanced cases, these lymphomas can present with severe lactic
tor, which plays a prominent role in many types of lymphomas. 95,96
Kaushansky_chapter 14_p0191-0202.indd 199 17/09/15 6:36 pm