Page 273 - Williams Hematology ( PDFDrive )
P. 273
248 Part IV: Molecular and Cellular Hematology Chapter 17: Signal Transduction Pathways 249
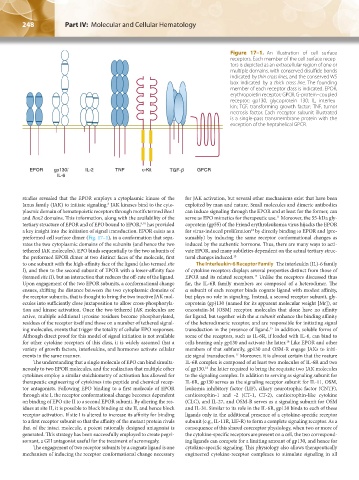
Figure 17–1. An illustration of cell surface
receptors. Each member of the cell surface recep-
tors is depicted as an extracellular region of one or
multiple domains, with conserved disulfide bonds
indicated by thin cross lines, and the conserved WS
box indicated by a thick cross line. The founding
member of each receptor class is indicated. EPOR,
erythropoietin receptor; GPCR, G-protein–coupled
receptor; gp130, glycoprotein 130; IL, interleu-
kin; TGF, transforming growth factor; TNF, tumor
necrosis factor. Each receptor subunit illustrated
is a single-pass transmembrane protein with the
exception of the heptahelical GPCR.
studies revealed that the EPOR employs a cytoplasmic kinase of the for JAK activation, but several other mechanisms exist that have been
Janus family (JAK) to initiate signaling. JAK kinases bind to the cyto- exploited by man and nature. Small molecules and dimeric antibodies
8
plasmic domain of hematopoietic receptors through motifs termed Box1 can induce signaling through the EPOR and at least for the former, can
and Box2 domains. This information, along with the availability of the serve as EPO mimetics for therapeutic use. Moreover, the 55-kDa gly-
11
tertiary structure of EPOR and of EPO bound to EPOR, has provided coprotein (gp55) of the Friend erythroleukemia virus hijacks the EPOR
9,10
a key insight into the initiation of signal transduction. EPOR exists as a for virus-induced proliferation by directly binding to EPOR and (pre-
12
preformed cell surface dimer (Fig. 17–1), in a conformation that sepa- sumably) by inducing the same receptor conformational changes as
rates the two cytoplasmic domains of the subunits (and hence the two induced by the authentic hormone. Thus, there are many ways to acti-
tethered JAK molecules). EPO binds sequentially to the two subunits of vate EPOR, and many subtleties dependent on the actual tertiary struc-
the preformed EPOR dimer at two distinct faces of the molecule, first tural changes induced. 13
to one subunit with the high-affinity face of the ligand (also termed site The Interleukin-6 Receptor Family The interleukin (IL)-6 family
I), and then to the second subunit of EPOR with a lower-affinity face of cytokine receptors displays several properties distinct from those of
(termed site II), but an interaction that reduces the off-rate of the ligand. EPOR and its related receptors. Unlike the receptors discussed thus
14
Upon engagement of the two EPOR subunits, a conformational change far, the IL-6R family members are composed of a heterodimer. The
ensues, shifting the distance between the two cytoplasmic domains of α subunit of each receptor binds cognate ligand with modest affinity,
the receptor subunits, that is thought to bring the two inactive JAK mol- but plays no role in signaling. Instead, a second receptor subunit, gly-
ecules into sufficiently close juxtaposition to allow cross-phosphoryla- coprotein (gp)130 (named for its apparent molecular weight [Mr]), or
tion and kinase activation. Once the two tethered JAK molecules are oncostatin-M (OSM) receptor, molecules that alone have no affinity
active, multiple additional tyrosine residues become phosphorylated, for ligand, but together with the α subunit enhance the binding affinity
residues of the receptor itself and those on a number of tethered signal- of the heterodimeric receptor, and are responsible for initiating signal
ing molecules, events that trigger the totality of cellular EPO responses. transduction in the presence of ligand. In addition, soluble forms of
15
Although direct proof for this model of signal initiation is not available some of the receptors, such as IL-6R, if loaded with IL-6, can bind to
for other cytokine receptors of this class, it is widely assumed that a cells bearing only gp130 and activate the latter. Like EPOR and other
16
variety of growth factors, interleukins, and hormones activate cellular members of that subfamily, gp130 and OSM-R engage JAKs to initi-
events in the same manner. ate signal transduction. Moreover, it is almost certain that the mature
17
The understanding that a single molecule of EPO can bind simulta- IL-6R complex is composed of at least two molecules of IL-6R and two
neously to two EPOR molecules, and the realization that multiple other of gp130, the latter required to bring the requisite two JAK molecules
18
cytokines employ a similar stoichiometry of activation has allowed for to the signaling complex. In addition to serving as signaling subunit for
therapeutic engineering of cytokines into peptide and chemical recep- IL-6R, gp130 serves as the signaling receptor subunit for IL-11, OSM,
tor antagonists. Following EPO binding to a first molecule of EPOR leukemia inhibitory factor (LIF), ciliary neurotrophic factor (CNTF),
through site I, the receptor conformational change becomes dependent cardiotrophin-1 and -2 (CT-1, CT-2), cardiotrophin-like cytokine
on binding of EPO site II to a second EPOR subunit. By altering the res- (CLC), and IL-27, and OSM-R serves as a signaling subunit for OSM
idues at site II, it is possible to block binding at site II, and hence block and IL-31. Similar to its role in the IL-6R, gp130 binds to each of these
receptor activation. If site I is altered to increase its affinity for binding ligands only in the additional presence of a cytokine-specific receptor
to a first receptor subunit so that the affinity of the mutant protein rivals subunit (e.g., IL-11R, LIF-R) to form a complete signaling receptor. As a
that of the intact molecule, a potent rationally designed antagonist is consequence of this shared coreceptor physiology, when two or more of
generated. This strategy has been successfully employed to create pegvi- the cytokine-specific receptors are present on a cell, the two correspond-
somant, a GH antagonist useful for the treatment of acromegaly. ing ligands can compete for a limiting amount of gp130, and hence for
The engagement of two receptor subunits by a cognate ligand is one cytokine-specific signaling. This physiology also allows therapeutically
mechanism of inducing the receptor conformational change necessary engineered cytokine-receptor complexes to stimulate signaling in all
Kaushansky_chapter 17_p0247-0256.indd 248 9/17/15 5:45 PM