Page 357 - Williams Hematology ( PDFDrive )
P. 357
332 Part V: Therapeutic Principles Chapter 22: Pharmacology and Toxicity of Antineoplastic Drugs 333
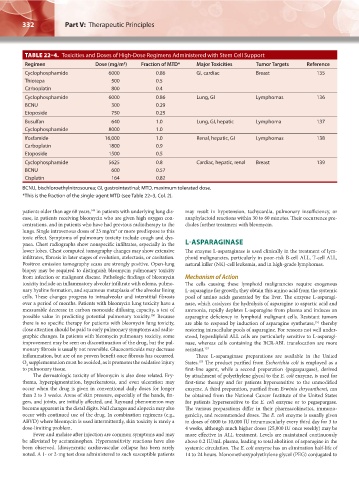
TABLE 22–4. Toxicities and Doses of High-Dose Regimens Administered with Stem Cell Support
Regimen Dose (mg/m ) Fraction of MTD* Major Toxicities Tumor Targets Reference
2
Cyclophosphamide 6000 0.86 GI, cardiac Breast 135
Thiotepa 500 0.5
Carboplatin 800 0.4
Cyclophosphamide 6000 0.86 Lung, GI Lymphomas 136
BCNU 300 0.29
Etoposide 750 0.25
Busulfan 640 1.0 Lung, GI, hepatic Lymphoma 137
Cyclophosphamide 8000 1.0
Ifosfamide 16,000 1.0 Renal, hepatic, GI Lymphomas 138
Carboplatin 1800 0.9
Etoposide 1500 0.5
Cyclophosphamide 5625 0.8 Cardiac, hepatic, renal Breast 139
BCNU 600 0.57
Cisplatin 164 0.82
BCNU, bischloroethylnitrosourea; GI, gastrointestinal; MTD, maximum tolerated dose.
*This is the fraction of the single-agent MTD (see Table 22–3, Col. 2).
patients older than age 60 years, in patients with underlying lung dis- may result in hypotension, tachycardia, pulmonary insufficiency, or
149
ease, in patients receiving bleomycin who are given high oxygen con- anaphylactoid reactions within 30 to 60 minutes. Their occurrence pre-
centrations, and in patients who have had previous radiotherapy to the cludes further treatment with bleomycin.
lungs. Single intravenous doses of 25 mg/m or more predispose to this
2
toxic effect. Symptoms of pulmonary toxicity include cough and dys-
pnea. Chest radiographs show nonspecific infiltrates, especially in the L-ASPARAGINASE
lower lobes. Chest computed tomography changes may show extensive The enzyme L-asparaginase is used clinically in the treatment of lym-
infiltrates, fibrosis in later stages of evolution, atelectasis, or cavitation. phoid malignancies, particularly in poor-risk B-cell ALL, T-cell ALL,
Positron emission tomography scans are strongly positive. Open-lung natural killer (NK)-cell leukemia, and in high-grade lymphomas.
biopsy may be required to distinguish bleomycin pulmonary toxicity
from infection or malignant disease. Pathologic findings of bleomycin Mechanism of Action
toxicity include an inflammatory alveolar infiltrate with edema, pulmo- The cells causing these lymphoid malignancies require exogenous
nary hyaline formation, and squamous metaplasia of the alveolar lining L-asparagine for growth; they obtain this amino acid from the systemic
cells. These changes progress to intraalveolar and interstitial fibrosis pool of amino acids generated by the liver. The enzyme L-asparagi-
over a period of months. Patients with bleomycin lung toxicity have a nase, which catalyzes the hydrolysis of asparagine to aspartic acid and
measurable decrease in carbon monoxide diffusing capacity, a test of ammonia, rapidly depletes L-asparagine from plasma and induces an
possible value in predicting potential pulmonary toxicity. Because asparagine deficiency in lymphoid malignant cells. Resistant tumors
150
there is no specific therapy for patients with bleomycin lung toxicity, are able to respond by induction of asparagine synthetase, thereby
151
close attention should be paid to early pulmonary symptoms and radio- restoring intracellular pools of asparagine. For reasons not well under-
graphic changes. In patients with bleomycin pulmonary toxicity, some stood, hyperdiploid ALL cells are particularly sensitive to L-asparagi-
improvement may be seen on discontinuation of the drug, but the pul- nase, whereas cells containing the BCR-ABL translocation are more
monary fibrosis is usually not reversible. Glucocorticoids may decrease resistant. 152
inflammation, but are of no proven benefit once fibrosis has occurred. Three L-asparaginase preparations are available in the United
O supplementation must be avoided, as it promotes the oxidative injury States. The product purified from Escherichia coli is employed as a
153
2
to pulmonary tissue. first-line agent, while a second preparation (pegaspargase), derived
The dermatologic toxicity of bleomycin is also dose related. Ery- by attachment of polyethylene glycol to the E. coli enzyme, is used for
thema, hyperpigmentation, hyperkeratosis, and even ulceration may first-time therapy and for patients hypersensitive to the unmodified
occur when the drug is given in conventional daily doses for longer enzyme. A third preparation, purified from Erwinia chrysanthemi, can
than 2 to 3 weeks. Areas of skin pressure, especially of the hands, fin- be obtained from the National Cancer Institute of the United States
gers, and joints, are initially affected, and Raynaud phenomenon may for patients hypersensitive to the E. coli enzyme or to pegaspargase.
become apparent in the distal digits. Nail changes and alopecia may also The various preparations differ in their pharmacokinetics, immuno-
occur with continued use of the drug. In combination regimens (e.g., genicity, and recommended doses. The E. coli enzyme is usually given
ABVD) where bleomycin is used intermittently, skin toxicity is rarely a in doses of 6000 to 10,000 IU intramuscularly every third day for 3 to
dose-limiting problem. 4 weeks, although much higher doses (25,000 IU once weekly) may be
Fever and malaise after injection are common symptoms and may more effective in ALL treatment. Levels are maintained continuously
be alleviated by acetaminophen. Hypersensitivity reactions have also above 0.2 IU/mL plasma, leading to total abolition of asparagine in the
been observed. Idiosyncratic cardiovascular collapse has been rarely systemic circulation. The E. coli enzyme has an elimination half-life of
noted. A 1- or 2-mg test dose administered to such susceptible patients 14 to 24 hours. Monomethoxypolyethylene glycol (PEG) conjugated to
Kaushansky_chapter 22_p0313-0352.indd 332 9/18/15 10:25 PM