Page 435 - Williams Hematology ( PDFDrive )
P. 435
410 Part V: Therapeutic Principles Chapter 26: Immune Cell Therapy 411
CD4+ Th cells are required for optimal CD8+ CTL responses and for controlling CMV infection after HSCT. However, the isolation
may eliminate CMV-infected cells that express class II MHC in vivo. and propagation of antigen-specific T-cell clones requires specific
Studies using recombinant CMV proteins or peptide panels have identi- expertise and is time-consuming. Culture methods for enrichment
fied CD4+ T-cell responses to pp65, IE-1, glycoprotein B, and the major of polyclonal CMV-specific T cells circumvent prolonged ex vivo
capsid protein (UL86) in normal CMV+ individuals. 10,24,25 manipulation and T-cell expansion and enabled broader application
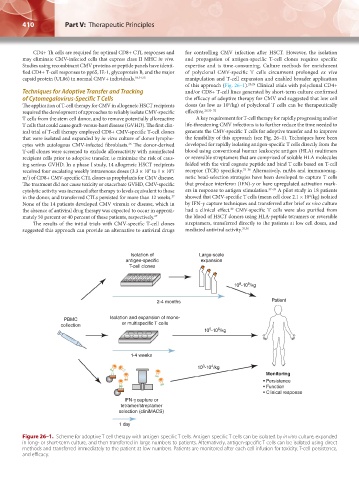
of this approach (Fig. 26–1). 28,29 Clinical trials with polyclonal CD4+
Techniques for Adoptive Transfer and Tracking and/or CD8+ T-cell lines generated by short-term culture confirmed
of Cytomegalovirus-Specific T Cells the efficacy of adoptive therapy for CMV and suggested that low cell
5
The application of T-cell therapy for CMV in allogeneic HSCT recipients doses (as low as 10 /kg) of polyclonal T cells can be therapeutically
required the development of approaches to reliably isolate CMV-specific effective. 28,30–32
T cells from the stem cell donor, and to remove potentially alloreactive A key requirement for T-cell therapy for rapidly progressing and/or
T cells that could cause graft-versus-host disease (GVHD). The first clin- life-threatening CMV infections is to further reduce the time needed to
ical trial of T-cell therapy employed CD8+ CMV-specific T-cell clones generate the CMV-specific T cells for adoptive transfer and to improve
that were isolated and expanded by in vitro culture of donor lympho- the feasibility of this approach (see Fig. 26–1). Techniques have been
26
cytes with autologous CMV-infected fibroblasts. The donor-derived developed for rapidly isolating antigen-specific T cells directly from the
T-cell clones were screened to exclude alloreactivity with noninfected blood using conventional human leukocyte antigen (HLA) multimers
recipient cells prior to adoptive transfer, to minimize the risk of caus- or reversible streptamers that are comprised of soluble HLA molecules
ing serious GVHD. In a phase I study, 14 allogeneic HSCT recipients folded with the viral cognate peptide and bind T cells based on T-cell
7
9
received four escalating weekly intravenous doses (3.3 × 10 to 1 × 10 / receptor (TCR) specificity. 33–36 Alternatively, mAbs and immunomag-
2
m ) of CD8+ CMV-specific CTL clones as prophylaxis for CMV disease. netic bead-selection strategies have been developed to capture T cells
The treatment did not cause toxicity or exacerbate GVHD, CMV-specific that produce interferon (IFN)-γ or have upregulated activation mark-
cytolytic activity was increased after therapy to levels equivalent to those ers in response to antigen stimulation. 37–39 A pilot study in 18 patients
4
27
in the donor, and transferred CTLs persisted for more than 12 weeks. showed that CMV-specific T cells (mean cell dose 2.1 × 10 /kg) isolated
None of the 14 patients developed CMV viremia or disease, which in by IFN-γ capture techniques and transferred after brief ex vivo culture
40
the absence of antiviral drug therapy was expected to occur in approxi- had a clinical effect. CMV-specific T cells were also purified from
mately 50 percent or 40 percent of these patients, respectively. 27 the blood of HSCT donors using HLA-peptide tetramers or reversible
The results of the initial trials with CMV-specific T-cell clones streptamers, transferred directly to the patients at low cell doses, and
suggested this approach can provide an alternative to antiviral drugs mediated antiviral activity. 35,36
Isolation of Large-scale
antigen-specific expansion
T-cell clones
9
8
10 -10 /kg
2-4 months Patient
PBMC Isolation and expansion of mono-
collection or multispecific T cells
6
5
10 -10 /kg
1-4 weeks
3
4
10 -10 /kg
Monitoring
• Persistence
• Function
• Clinical response
IFN-γ capture or
tetramer/streptamer
selection (cliniMACS)
1 day
Figure 26–1. Scheme for adoptive T-cell therapy with antigen-specific T cells. Antigen-specific T cells can be isolated by in vitro culture, expanded
in long- or short-term culture, and then transferred in large numbers to patients. Alternatively, antigen-specific T cells can be isolated using direct
methods and transferred immediately to the patient at low numbers. Patients are monitored after each cell infusion for toxicity, T-cell persistence,
and efficacy.
Kaushansky_chapter 26_p0409-0420.indd 410 9/17/15 6:01 PM