Page 449 - Williams Hematology ( PDFDrive )
P. 449
424 Part V: Therapeutic Principles Chapter 27: Vaccine Therapy 425
50 One strategy is to vaccinate patients after chemotherapy, when a state
of minimal residual disease has been achieved. Moreover, lymphope-
UPN Postvaccine
nia induced by chemotherapy can also enhance antitumor immunity by
40 6 promoting expansion of vaccine-induced effector T cells and minimiz-
33
8 ing tumor-induced immune suppression. In this setting, the profound
15
lymphopenia from chemotherapy results in release of cytokines, such as
Specific lysis (%) 30 16 IL-15, providing a powerful proliferative drive to lymphocytes, which
19
could be exploited to increase specific lymphocyte responses to the
23
vaccine. Moreover, this strategy can eradicate cells that suppress antitu-
mor responses such as regulatory T cells, and may overcome inherent
20
defects in T-cell signaling, processing, or presentation. In addition,
33
the afferent phase of the immune response could be enhanced by using
10 more potent vaccines with novel adjuvants such as toll-like receptor
ligands, or by vaccinating donors of transplant recipients who have a
Maximum level healthy immune system as opposed to patients who may be immuno-
reached prevaccine
41
0 compromised either from the cancer or from therapy. Alternatively,
0 12.5 25 50 100 vaccines could be used in combination with agents that inhibit immu-
Effector-to-target ratio nosuppressive mechanisms, such as immune checkpoint receptors/lig-
43
42
ands and/or deplete regulatory T cells, to augment the afferent and/
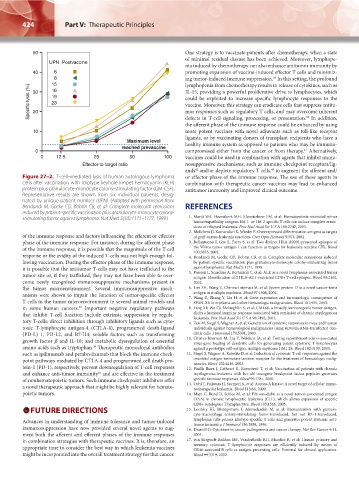
Figure 27–2. T-cell–mediated lysis of human autologous lymphoma or effector phase of the immune response. The use of these agents in
cells after vaccination with idiotype keyhole limpet hemocyanin (KLH) combination with therapeutic cancer vaccines may lead to enhanced
protein plus granulocyte-monocyte colony-stimulating factor (GM-CSF). antitumor immunity and improved clinical outcome.
Representative results are shown from six individual patients, desig-
nated by unique patient number (UPN). (Adapted with permission from
Bendandi M, Gocke CD, Kobrin CB, et al: Complete molecular remissions REFERENCES
induced by patient-specific vaccination plus granulocyte-monocyte colony-
stimulating factor against lymphoma. Nat Med 5(10):1171–1177, 1999.) 1. Marijt WA, Heemskerk MH, Kloosterboer FM, et al: Hematopoiesis-restricted minor
histocompatibility antigens HA-1- or HA-2-specific T cells can induce complete remis-
sions of relapsed leukemia. Proc Natl Acad Sci U S A 100:2742, 2003.
2. Molldrem JJ, Komanduri K, Wieder E: Overexpressed differentiation antigens as targets
of the immune response and factors influencing the efferent or effector of graft-versus-leukemia reactions. Curr Opin Hematol 9:503, 2002.
phase of the immune response. For instance, during the afferent phase 3. Bellantuono I, Gao L, Parry S, et al: Two distinct HLA-A0201-presented epitopes of
of the immune response, it is possible that the magnitude of the T-cell the Wilms tumor antigen 1 can function as targets for leukemia-reactive CTL. Blood
100:3835, 2002.
response or the avidity of the induced T-cells was not high enough fol- 4. Bendandi M, Gocke CD, Kobrin CB, et al: Complete molecular remissions induced
lowing vaccination. During the effector phase of the immune response, by patient-specific vaccination plus granulocyte-monocyte colony-stimulating factor
it is possible that the antitumor T-cells may not have trafficked to the against lymphoma. Nat Med 5:1171, 1999.
tumor site or, if they trafficked, they may not have been able to over- 5. Passoni L, Scardino A, Bertazzoli C, et al: ALK as a novel lymphoma-associated tumor
antigen: Identification of 2 HLA-A2.1-restricted CD8+ T-cell epitopes. Blood 99:2100,
come newly recognized immunosuppressive mechanisms present in 2002.
the tumor microenvironment. Several immunosuppressive mech- 6. Lim SH, Wang Z, Chiriva-Internati M, et al: Sperm protein 17 is a novel cancer-testis
anisms were shown to impair the function of tumor-specific effector antigen in multiple myeloma. Blood 97:1508, 2001.
T cells in the tumor microenvironment in several animal models and 7. Wang Z, Zhang Y, Liu H, et al: Gene expression and immunologic consequence of
SPAN-Xb in myeloma and other hematologic malignancies. Blood 101:955, 2003.
in some human cancers. Important negative regulatory pathways 8. Yang XF, Wu CJ, Mclaughlin S, et al: CML66, a broadly immunogenic tumor antigen,
39
that inhibit T-cell function include extrinsic suppression by regula- elicits a humoral immune response associated with remission of chronic myelogenous
leukemia. Proc Natl Acad Sci U S A 98:7492, 2001.
tory T-cells; direct inhibition through inhibitory ligands such as cyto- 9. Zeis M, Siegel S, Wagner A, et al: Generation of cytotoxic responses in mice and human
toxic T-lymphocyte antigen-4 (CTLA-4), programmed death-ligand individuals against hematological malignancies using survivin-RNA-transfected den-
(PD-L) 1, PD-L2, and B7-H4; soluble factors such as transforming dritic cells. J Immunol 170:5391, 2003.
growth factor β and IL-10; and metabolic dysregulation of essential 10. Chiriva-Internati M, Liu Y, Weidanz JA, et al: Testing recombinant adeno-associated
amino acids such as tryptophan. Therapeutic monoclonal antibodies virus-gene loading of dendritic cells for generating potent cytotoxic T lymphocytes
39
against a prototype self-antigen, multiple myeloma HM1.24. Blood 102:3100, 2003.
such as ipilimumab and pembrolizumab that block the immune check- 11. Siegel S, Wagner A, Kabelitz D, et al: Induction of cytotoxic T-cell responses against the
point pathways mediated by CTLA-4 and programmed cell death pro- oncofetal antigen-immature laminin receptor for the treatment of hematologic malig-
nancies. Blood 102:4416, 2003.
tein-1 (PD-1), respectively, prevent downregulation of T-cell responses 12. Pinilla-Ibarz J, Cathcart K, Korontsvit T, et al: Vaccination of patients with chronic
and enhance anti-tumor immunity and are effective in the treatment myelogenous leukemia with bcr-abl oncogene breakpoint fusion peptides generates
40
of nonhematopoietic tumors. Such immune checkpoint inhibitors offer specific immune responses. Blood 95:1781, 2000.
a novel therapeutic approach that might be highly relevant for hemato- 13. Ochi T, Fujiwara H, Suemori K, et al: Aurora-A kinase: A novel target of cellular immu-
notherapy for leukemia. Blood 113:66, 2009.
poietic tumors. 14. Mayr C, Bund D, Schlee M, et al: Fibromodulin as a novel tumor-associated antigen
(TAA) in chronic lymphocytic leukemia (CLL), which allows expansion of specific
CD8+ autologous T lymphocytes. Blood 105:1566, 2005.
FUTURE DIRECTIONS 15. Levitsky HI, Montgomery J, Ahmadzadeh M, et al: Immunization with granulo-
cyte-macrophage colony-stimulating factor-transduced, but not B7–1-transduced,
Advances in understanding of immune tolerance and tumor-induced lymphoma cells primes idiotype-specific T cells and generates potent systemic anti-
tumor immunity. J Immunol 156:3858, 1996.
immunosuppression have now provided several novel agents to aug- 16. Dranoff G: Cytokines in cancer pathogenesis and cancer therapy. Nat Rev Cancer 4:11,
ment both the afferent and efferent phases of the immune responses 2004.
in combination strategies with therapeutic vaccines. It is, therefore, an 17. von Bergwelt-Baildon MS, Vonderheide RH, Maecker B, et al: Human primary and
appropriate time to consider the best way in which leukemia vaccines memory cytotoxic T lymphocyte responses are efficiently induced by means of
CD40-activated B cells as antigen-presenting cells: Potential for clinical application.
might be incorporated into the overall treatment strategy for that cancer. Blood 99:3319, 2002.
Kaushansky_chapter 27_p0421-0426.indd 424 9/17/15 6:02 PM