Page 448 - Williams Hematology ( PDFDrive )
P. 448
422 Part V: Therapeutic Principles Chapter 27: Vaccine Therapy 423
TABLE 27–2. Monitoring of Human Immune Responses 100 Idiotype-KLH + GM-CSF
Type of Response Representative Assay Days 1–4 20,000 units
10,000 units
SC
CD4+ T cells Cytokine induction ldiotype-KLH
80 alone
Cytokine ELISPOT (IFN-γ) Control idiotype-KLH
Intracellular cytokine 60 Mantel-Cox P-values
Proliferation % Survival vs. 0.15
CD8+ T cells Cytotoxicity vs. 0.01
Limiting dilution analysis 40
Tetramer
20
Cytokine ELISPOT (IFN-γ)
Intracellular cytokine
0
Antibody ELISA 10 20 30 40
Flow cytometry Days posttumor challenge
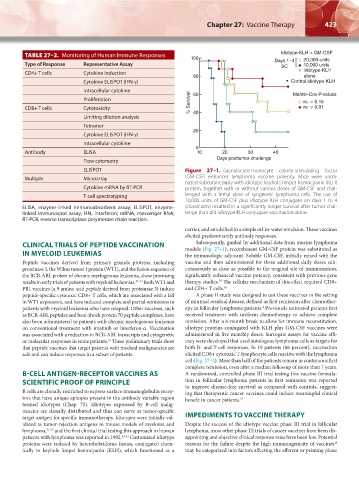
ELISPOT Figure 27–1. Granulocyte-monocyte colony-stimulating factor
Multiple Microarray (GM-CSF) enhances lymphoma vaccine potency. Mice were vacci-
nated subcutaneously with idiotype keyhole limpet hemocyanin (KLH)
Cytokine mRNA by RT-PCR protein, together with or without various doses of GM-CSF and chal-
T-cell spectratyping lenged with a lethal dose of syngeneic lymphoma cells. The use of
10,000 units of GM-CSF plus idiotype KLH conjugate on days 1 to 4
ELISA, enzyme-linked immunoabsorbent assay; ELISPOT, enzyme- (closed dots) resulted in a significantly longer survival after tumor chal-
linked immunospot assay; IFN, interferon; mRNA, messenger RNA; lenge than did idiotype KLH conjugate vaccination alone.
RT-PCR, reverse transcriptase polymerase chain reaction.
carrier, and emulsified in a simple oil-in-water emulsion. These vaccines
elicited predominantly antibody responses.
Subsequently, guided by additional data from murine lymphoma
CLINICAL TRIALS OF PEPTIDE VACCINATION models (Fig. 27–1), recombinant GM-CSF protein was substituted as
IN MYELOID LEUKEMIAS the immunologic adjuvant. Soluble GM-CSF, initially mixed with the
Peptide vaccines derived from primary granule proteins, including vaccine and then administered for three additional daily doses sub-
proteinase 3, the Wilms tumor 1 protein (WT1), and the fusion sequence of cutaneously as close as possible to the original site of immunization,
the BCR-ABL protein of chronic myelogenous leukemia, show promising significantly enhanced vaccine potency, consistent with previous gene
results in early trials of patients with myeloid leukemias. 27–31 Both WT1 and therapy studies. The cellular mechanism of this effect required CD8+
35
PR1 vaccines (a 9 amino acid peptide derived from proteinase 3) induce and CD4+ T cells. 36
peptide-specific cytotoxic CD8+ T cells, which are associated with a fall A phase II study was designed to test these vaccines in the setting
in WT1 expression, and have induced complete and partial remissions in of minimal residual disease, defined as first remission after chemother-
patients with myeloid leukemia who have relapsed. Other vaccines, such apy in follicular lymphoma patients. Previously untreated patients first
4
as BCR-ABL peptides and heat-shock protein 70 peptide complexes, have received treatment with uniform chemotherapy to achieve complete
also been administered to patients with chronic myelogenous leukemia remission. After a 6-month break to allow for immune reconstitution,
on conventional treatment with imatinib or interferon-α. Vaccination idiotype proteins conjugated with KLH plus GM-CSF vaccines were
was associated with a reduction in BCR-ABL transcripts and cytogenetic administered in five monthly doses. Surrogate assays for vaccine effi-
or molecular responses in some patients. These preliminary trials show cacy were developed that used autologous lymphoma cells as targets for
31
that peptide vaccines that target patients with myeloid malignancies are both B- and T-cell responses. In 19 patients (86 percent), vaccination
safe and can induce responses in a subset of patients. elicited CD8+ cytotoxic T-lymphocyte cells reactive with the lymphoma
cell (Fig. 27–2). More than half of the patients remain in continuous first
complete remission, even after a median followup of more than 7 years.
B-CELL ANTIGEN-RECEPTOR VACCINES AS A randomized, controlled phase III trial testing this vaccine formula-
SCIENTIFIC PROOF OF PRINCIPLE tion in follicular lymphoma patients in first remission was reported
to improve disease-free survival as compared with controls, suggest-
B cells are clonally restricted to express surface immunoglobulin recep- ing that therapeutic cancer vaccines could induce meaningful clinical
tors that have unique epitopes present in the antibody variable region benefit in cancer patients. 37
termed idiotypes (Chap. 75). Idiotypes expressed by B-cell malig-
nancies are clonally distributed and thus can serve as tumor-specific
target antigen for specific immunotherapy. Idiotypes were initially val- IMPEDIMENTS TO VACCINE THERAPY
idated as tumor-rejection antigens in mouse models of myeloma and Despite the success of the idiotype vaccine phase III trial in follicular
lymphoma, 31,32 and the first clinical trial testing this approach in human lymphoma, most other phase III trials of cancer vaccines have been dis-
patients with lymphoma was reported in 1992. 33,34 Customized idiotype appointing and objective clinical response rates have been low. Potential
38
proteins were isolated by heterohybridoma fusion, conjugated chem- reasons for the failure despite the high immunogenicity of vaccines
ically to keyhole limpet hemocyanin (KLH), which functioned as a may be categorized into factors affecting the afferent or priming phase
Kaushansky_chapter 27_p0421-0426.indd 423 9/17/15 6:02 PM