Page 535 - Williams Hematology ( PDFDrive )
P. 535
510 Part VI: The Erythrocyte Chapter 34: Clinical Manifestations and Classification of Erythrocyte Disorders 511
loss-of-function PHD2 variants (encoded by EGLN1 gene) have normal
O transport (Cardiac output x Hct) EPO levels.
2
Because of the rarity of HIF2a and PHD2 mutations, their non-
erythroid phenotype is not well defined; however, some HIF2a muta-
Severe tions, possibly associated with genetic mosaicism, are associated with
hypervolemia
4 congenital polycythemia and later development of pheochromocytoma/
paraganglioma and somatistatinoma tumors. In these instances, in most
patients, the HIF2a mutation is present in the tumors, which frequently
Oxygen transport 1 3 hypervolemia recur, but not in leukocytes. 44,45
2
Moderate
REFERENCES
1. Semenza GL, Nejfelt MK, Chi SM, et al: Hypoxia-inducible nuclear factors bind to an
Normovolemia enhancer element located 3′ to the human erythropoietin gene. Proc Natl Acad Sci U S A
88(13):5680, 1991.
2. Guillemin K, Krasnow MA: The hypoxic response: Huffing and HIFing. Cell 89(1):9,
1997.
3. Hochachka PW, Buck LT, Doll CJ, et al: Unifying theory of hypoxia tolerance: Molecular/
metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci
U S A 93(18):9493, 1996.
4. Semenza GL: O -regulated gene expression: Transcriptional control of cardiorespira-
2
tory physiology by HIF-1. J Appl Physiol (1985) 96(3):1173; discussion 1170, 2004.
0 20 40 60 80 100 5. Srinivas V, Zhu X, Salceda S, et al: Hypoxia-inducible factor 1alpha (HIF-1alpha) is a non
Hematocrit (%) -heme iron protein. Implications for oxygen sensing. J Biol Chem 273(29):18019, 1998.
6. Ivan M, Kondo K, Yang H, et al: HIFalpha targeted for VHL-mediated destruction by
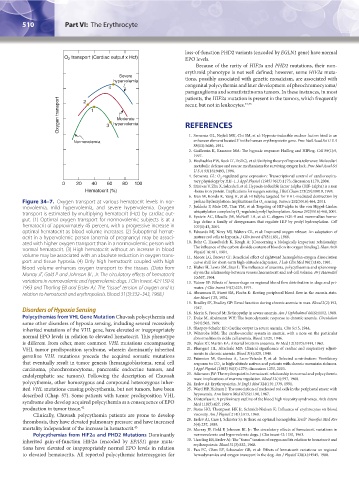
Figure 34–7. Oxygen transport at various hematocrit levels in nor- proline hydroxylation: Implications for O sensing. Science 292(5516):464, 2001.
2
movolemia, mild hypervolemia, and severe hypervolemia. Oxygen 7. Jaakkola P, Mole DR, Tian YM, et al: Targeting of HIF-alpha to the von Hippel-Lindau
transport is estimated by multiplying hematocrit (Hct) by cardiac out- ubiquitylation complex by O -regulated prolyl hydroxylation. Science 292(5516):468, 2001.
2
put. (1) Optimal oxygen transport for normovolemic subjects is at a 8. Epstein AC, Gleadle JM, McNeill LA, et al: C. elegans EGL-9 and mammalian homo-
logs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell
hematocrit of approximately 45 percent, with a progressive increase in 107(1):43, 2001.
optimal hematocrit as blood volume increases. (2) Suboptimal hemat- 9. Edwards MJ, Novy MJ, Walters CL, et al: Improved oxygen release: An adaptation of
ocrit in a hypervolemic person (anemia of pregnancy) may be associ- mature red cells to hypoxia. J Clin Invest 47(8):1851, 1968.
ated with higher oxygen transport than in a normovolemic person with 10. Bohr C, Hasselbalch K, Krogh A: [Concerning a biologically important relationship:
normal hematocrit. (3) High hematocrit without an increase in blood The influence of the carbon dioxide content of blood on its oxygen binding]. Skan Arch
Physiol 16:401, 1904.
volume may be associated with an absolute reduction in oxygen trans- 11. Moore LG, Brewer GJ: Beneficial effect of rightward hemoglobin-oxygen dissociation
port and tissue hypoxia. (4) Only high hematocrit coupled with high curve shift for short-term high-altitude adaptation. J Lab Clin Med 98(1):145, 1981.
blood volume enhances oxygen transport to the tissues. (Data from 12. Huber H, Lewis SM, Szur L: The influence of anaemia, polycythaemia and splenomeg-
Murray JF, Gold P and Johnson BL, Jr. The circulatory effects of hematocrit aly on the relationship between venous haematocrit and red-cell volume. Br J Haematol
10:567, 1964.
variations in normovolemic and hypervolemic dogs. J Clin Invest. 42:1150-9, 13. Vatner SF: Effects of hemorrhage on regional blood flow distribution in dogs and pri-
1963 and Thorling EB and Erslev AJ. The “tissue” tension of oxygen and its mates. J Clin Invest 54(2):225, 1974.
relation to hematocrit and erythropoiesis. Blood 31(3):332–343, 1968.) 14. Abramson D, Fierst SM, Flachs K: Resting peripheral blood flow in the anemia state.
Am Heart J 25, 1954.
15. Bradley SE, Bradley GP: Renal function during chronic anemia in man. Blood 2(2):192,
Disorders of Hypoxia Sensing 1947.
Polycythemias from VHL Gene Mutation Chuvash polycythemia and 16. Merin S, Freund M: Retinopathy in severe anemia. Am J Ophthalmol 66(6):1102, 1968.
17. Duke M, Abelmann WH: The hemodynamic response to chronic anemia. Circulation
some other disorders of hypoxia sensing, including several recessively 39(4):503, 1969.
inherited mutations of the VHL gene, have elevated or inappropriately 18. Sharpey-Schafer EP: Cardiac output in severe anemia. Clin Sci 5, 1944.
normal EPO levels in relation to elevated hematocrit. This phenotype 19. Wintrobe MM: The cardiovascular system in anemia; with a note on the particular
abnormalities in sickle cell anemia. Blood 1:121, 1946.
is different from other, more common VHL mutations encompassing 20. Wales RT, Martin EA: Arterial bruits in anaemia. Br Med J 2(5370):1444, 1963.
VHL tumor predisposition syndrome, wherein dominantly inherited 21. Blumgart HL, Altschule MD: Clinical significance of cardiac and respiratory adjust-
ments in chronic anemia. Blood 3(4):329, 1948.
germline VHL mutations precede the acquired somatic mutations 22. Fatemian M, Gamboa A, Leon-Velarde F, et al: Selected contribution: Ventilatory
that eventually result in tumor genesis (hemangioblastoma, renal cell response to CO in high-altitude natives and patients with chronic mountain sickness.
2
carcinoma, pheochromocytoma, pancreatic endocrine tumors, and J Appl Physiol (1985) 94(3):1279; discussion 1253, 2003.
endolymphatic sac tumors). Following the description of Chuvash 23. Adamson JW: The erythropoietin-hematocrit relationship in normal and polycythemic
man: Implications of marrow regulation. Blood 32(4):597, 1968.
polycythemia, other homozygous and compound heterozygous inher- 24. Erslev AJ: Erythropoietin. N Engl J Med 324(19):1339, 1991.
ited VHL mutations causing polycythemia, but not tumors, have been 25. Ward HP, Holman J: The association of nucleated red cells in the peripheral smear with
described (Chap. 57). Some patients with tumor predisposition VHL hypoxemia. Ann Intern Med 67(6):1190, 1967.
syndrome also develop acquired polycythemia as a consequence of EPO 26. Dintenfass L: A preliminary outline of the blood high viscosity syndromes. Arch Intern
Med 118(5):427, 1966.
production in tumor tissue. 42 27. Stone HO, Thompson HK Jr, Schmidt-Nielsen K: Influence of erythrocytes on blood
Clinically, Chuvash polycythemia patients are prone to develop viscosity. Am J Physiol 214(4):913, 1968.
thrombosis, they have elevated pulmonary pressure and have increased 28. Erslev AJ, Caro J, Schuster SJ: Is there an optimal hemoglobin level? Transfus Med Rev
3(4):237, 1989.
mortality independent of the increase in hematocrit. 43 29. Murray JF, Gold P, Johnson BL Jr: The circulatory effects of hematocrit variations in
Polycythemias from HIF2α and PHD2 Mutations Dominantly normovolemic and hypervolemic dogs. J Clin Invest 42:1150, 1963.
inherited gain-of-function HIF2α (encoded by EPAS1) gene muta- 30. Thorling EB, Erslev AJ: The “tissue” tension of oxygen and its relation to hematocrit and
erythropoiesis. Blood 31(3):332, 1968.
tions have elevated or inappropriately normal EPO levels in relation 31. Fan FC, Chen RY, Schuessler GB, et al: Effects of hematocrit variations on regional
to elevated hematocrits. All reported polycythemic heterozygotes for hemodynamics and oxygen transport in the dog. Am J Physiol 238(4):H545, 1980.
Kaushansky_chapter 34_p0503-0512.indd 510 9/17/15 6:12 PM