Page 785 - Williams Hematology ( PDFDrive )
P. 785
760 Part VI: The Erythrocyte Chapter 49: Disorders of Hemoglobin Structure: Sickle Cell Anemia and Related Abnormalities 761
or δ). The α-chains of all human Hbs encountered after early embryo- molecules. Areas of contact between chains and between heme and glo-
genesis are the same. The non-α chains include the β-chain of normal bin tend to contain invariant residues. The non-α (β, γ, δ, or ε) chains
adult Hb (α β ), the γ-chain of fetal Hb (α γ ), and the δ-chain of the are all 146 amino acids in length. The γ-chain of fetal Hb (HbF) differs
2 2
2 2
minor adult Hb (HbA [α δ ]), which accounts for 2.5 percent of the Hb from the β-chain by 39 residues. The γ genes are duplicated: one codes
2
2 2
G
7
of normal adults. Chapter 48 discusses the regulation of production of for glycine ( γ) and the other for alanine ( γ) at residue 136, giving rise
A
the globin chains. to two kinds of γ chains. In addition, a common polymorphism, the
Certain residues in the amino acid sequence of each polypeptide substitution of threonine for isoleucine, is frequently found at residue
chain appear to be critical to stability and function. Such residues are 75 of the γ-chain.
A
usually the same (invariant) in α or β chains. The NH -terminal valines of Approximately 75 percent of the amino acids in α and β chains are
2
the β chains are important in 2,3-BPG interactions. The C-terminal res- in a helical arrangement. All Hbs studied have a similar helical content
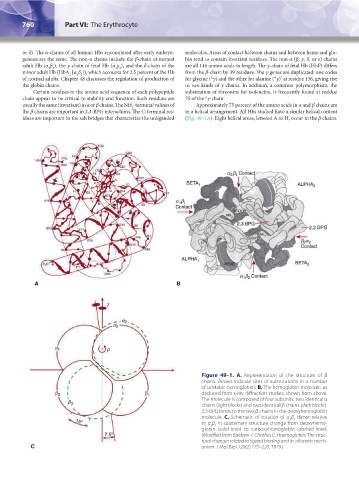
idues are important in the salt bridges that characterize the unliganded (Fig. 49–1A). Eight helical areas, lettered A to H, occur in the β chains.
Figure 49–1. A. Representation of the structure of β
chains. Arrows indicate sites of substitutions in a number
of unstable hemoglobins. B. The hemoglobin molecule, as
deduced from x-ray diffraction studies, shown from above.
The molecule is composed of four subunits: two identical α
chains (light blocks) and two identical β chains (dark blocks).
2,3-BPG binds to the two β chains in the deoxyhemoglobin
molecule. C. Schematic of rotation of α β dimer relative
2 2
to α β in quaternary structure change from deoxyhemo-
1 1
globin (solid lines) to carboxyhemoglobin (dashed lines).
(Modified from Baldwin J, Chothia C: Haemoglobin: The struc-
tural changes related to ligand binding and its allosteric mech-
anism. J Mol Biol 129(2):175–220, 1979.)
Kaushansky_chapter 49_p0759-0788.indd 760 9/18/15 3:01 PM